Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: DISCOVER 1 & 2, two double-blind, phase 3, psoriatic arthritis (PsA) trials of guselkumab (GUS, an IL-23 inhibitor), demonstrated significant improvement with GUS vs placebo (PBO) in signs and symptoms of PsA, with good tolerability, at week (w) 24 during the PBO-controlled period.1,2 Beyond w24, all patients switched to GUS. Continued treatment maintained efficacy through w52.3,4 Here, we describe pooled safety results from the DISCOVER 1 & 2 trials through 1-year of GUS treatment.
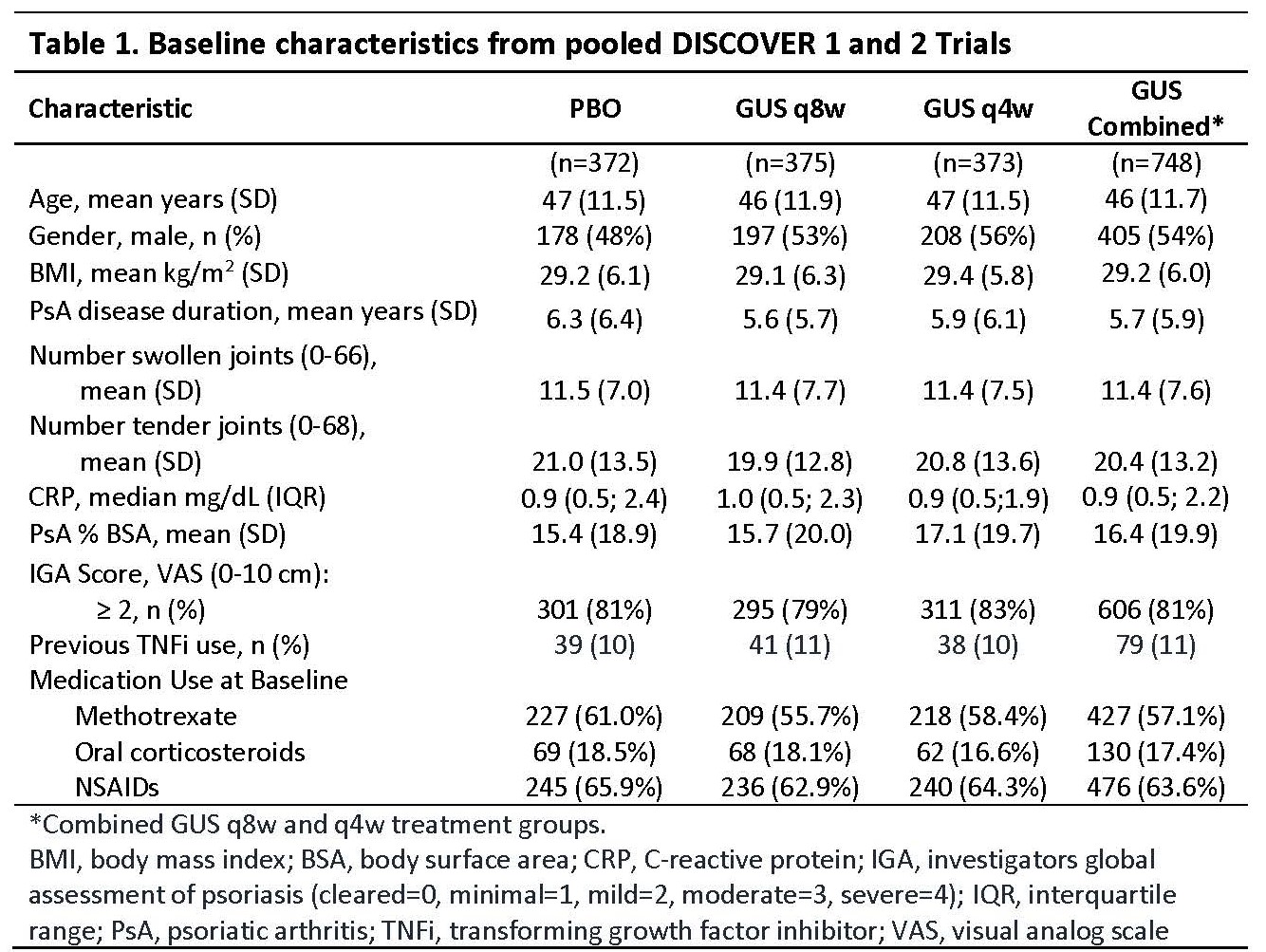
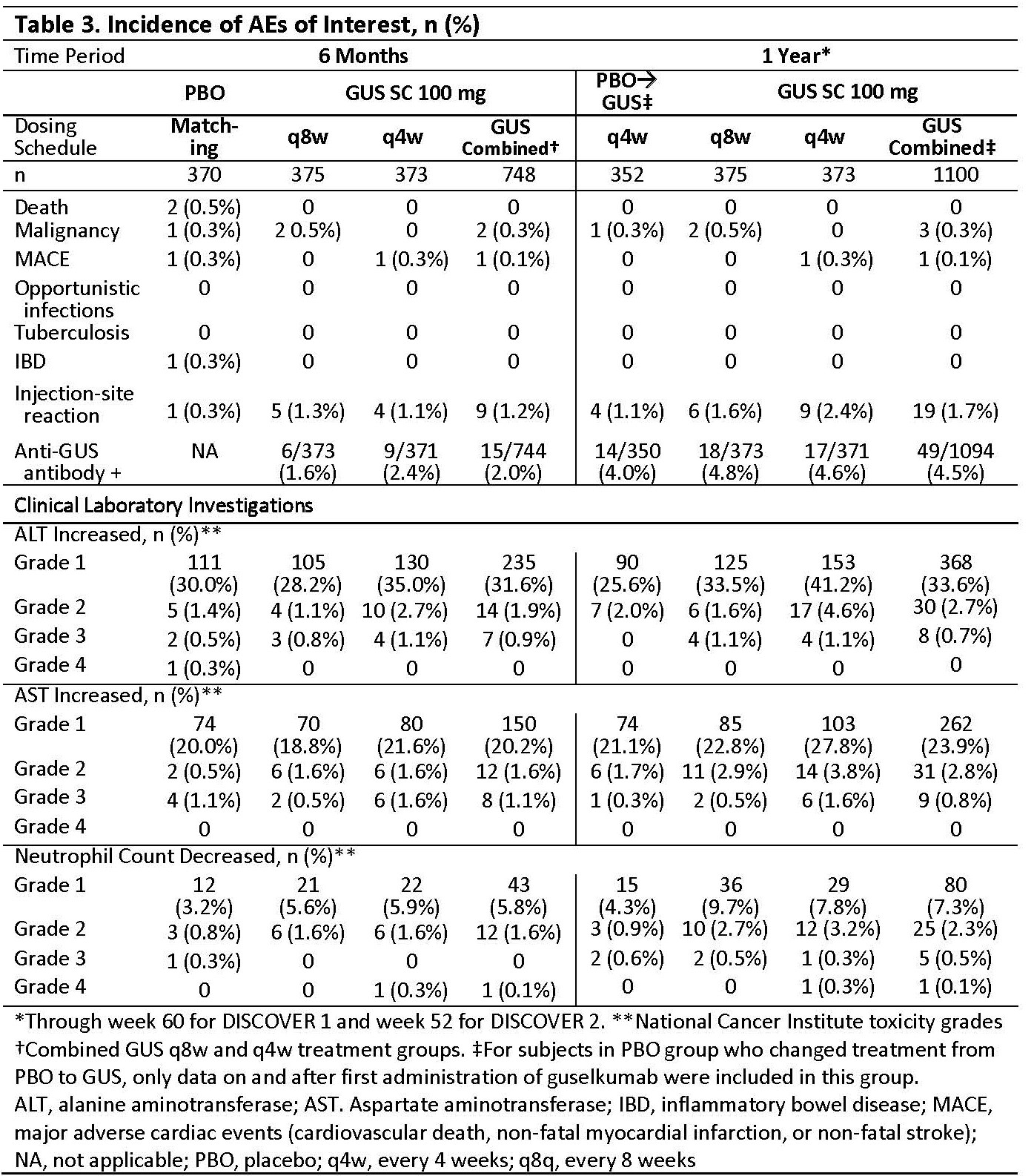
Methods: Adults with active PsA (≥3 tender/swollen joints and C-Reactive protein [CRP] ≥0.3 mg/dL in DISCOVER 1; ≥5 tender/swollen joints and CRP ≥0.6 mg/dL in DISCOVER 2) were enrolled. Patients were biologic naive except ~30% patients in DISCOVER 1 had taken 1-2 TNF inhibitors. Patients were randomized to subcutaneous GUS 100 mg at w0, w4, then every 8 w (q8w); GUS 100 mg q4w; or PBO. At w24, PBO patients were switched to GUS 100 mg q4w. Adverse events (AEs), laboratory investigations, and AEs of interest (Table 3) through w60 (end of trial) in DISCOVER 1 and through w52 in DISCOVER 2 were reported.
Results: Baseline characteristics were similar between treatment groups in the pooled studies (Table 1). Through w24 and 1 year, time adjusted results (per 100 patient years [PY]) for numbers of patients with ≥1 event were similar among treatment groups for AEs, serious AEs, infections, serious infections, and discontinuations due to an AE (Table 2). For events of interest at 1 year (Table 3), there were no cases of active tuberculosis or opportunistic infections (including Candida); no inflammatory bowel disease in GUS-treated patients; 2 deaths in PBO patients; and low incidences that were similar across treatment groups for malignancy, major adverse cardiac events, and injection-site reactions. Incidence of anti-GUS antibodies was 4.5%, and most were not neutralizing. Mild elevations in serum hepatic transaminases and decreases in neutrophil counts were consistent at 1 year with the results at w24 (Table 3).
Conclusion: GUS regimens of q8w and q4w were well tolerated in PsA patients through 1 year of treatment in the phase 3 DISCOVER trials, consistent with the w24 results. There were no meaningful differences between incidences of AEs reported in the q8w and q4w groups. The safety profile of GUS in PsA patients is generally comparable with the previously established safety profile of GUS.
References:
- Deodhar A et al. Lancet. 2020;395:1115.
- Mease P et al. Lancet. 2020;395:1126.
- Ritchlin C et al. EULAR 2020 # SAT0397.
- McInnes I et al. EULAR 2020 # SAT0402.
To cite this abstract in AMA style:
Ritchlin C, Rahman P, Helliwell P, Boehncke W, McInnes I, Gottlieb A, Kafka S, Kollmeier A, Hsia E, Xu X, Shawi M, Sheng S, Agarwal P, Zhou B, Ramachandran P, Mease P. Pooled Safety Results from Two Phase-3 Trials of Guselkumab in Patients with Psoriatic Arthritis Through 1 Year [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/pooled-safety-results-from-two-phase-3-trials-of-guselkumab-in-patients-with-psoriatic-arthritis-through-1-year/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pooled-safety-results-from-two-phase-3-trials-of-guselkumab-in-patients-with-psoriatic-arthritis-through-1-year/