Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The polysymptomatic distress (PSD) scale is derived from variables used in the 2010 American College of Rheumatology (ACR) fibromyalgia (FM) criteria as modified for survey and clinical research. The scale is useful in measuring the effect of PSD over the full range of human illness, not just in those who are ACR criteria positive. However, no PSD scale categories have been defined to distinguish severity of illness in FM or in those who do not satisfy criteria. We analyzed the scale and multiple covariates to develop useful clinical categories for PSD and to further validate the scale.
Methods: Fibromyalgia was diagnosed according to the research criteria modification of the 2010 ACR fibromyalgia criteria. We used the 2012 German general population survey (N=2445) to establish “normal” values. We then investigated categories in a large sample of patients with pain: 2,732 with rheumatoid arthritis (RA), and developed categories by utilizing germane clinic variables that had been previously studied for severity groupings. By ACR definition, FM cannot be diagnosed unless PSD is at least 12.
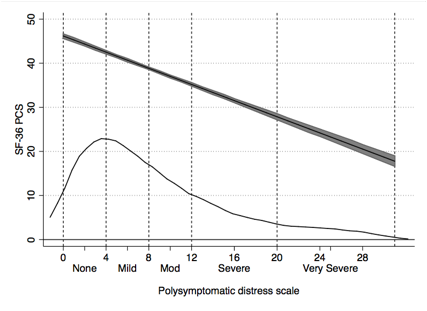
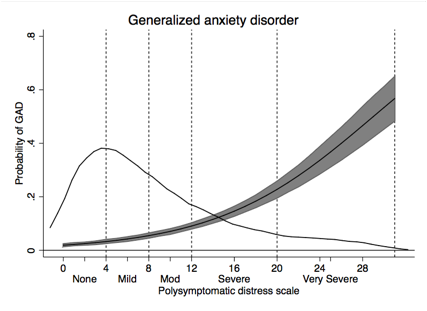
Results: The mean PSD of those with FM in the General population was 16.2 and was 19.2 in the RA clinical sample. Based on population categories and regression analysis and inspections of curvilinear relationships in RA, we established PSD severity categories of None (0-3), Mild (4-7), Moderate (8-11), Severe (12-19) and Very severe (20-31). Categories were statistically distinct, and a generally linear relationship between PSD categories and covariate severity was noted (Table and figures). The thin line in the figures represents the distribution of PSD values.
Conclusion: The described PSD categories are clinically relevant and demonstrate FM type symptoms over the full range of clinical illness, not just in FM positive subjects. Although FM criteria can be clinically useful, no clear-cut distinction between FM (+) and FM (-) subjects can be seen in our data.
Table 1. Clinical variables according to PSD severity groups.
|
PSD Group |
PSD (0-3) |
PSD (4-7) |
PSD (8-11) |
PSD FM (+) (12-19) FM (-) excluded |
PSD FM (+) (20-31) FM (-) excluded |
|
Pain (0-10) |
1.3 |
2.7 |
4 |
5.3 |
6.8 |
|
Global severity (0-10) |
1.4 |
2.8 |
3.9 |
5.2 |
6.3 |
|
HAQ (0-3) |
0.4 |
0.8 |
1.1 |
1.4 |
1.7 |
|
PCS score (SF-36) |
47.4 |
40.2 |
34.6 |
30.8 |
27.5 |
|
GAD Anxiety case (%) |
1.3 |
3.4 |
6.8 |
22.3 |
30.6 |
|
PHQ-2 Depression case (%) |
0.1 |
2.3 |
5.6 |
26.1 |
34.8 |
|
Regional Pain Scale (0-19) |
0.8 |
2.6 |
5 |
8.2 |
16.3 |
|
Symptom severity (0-12)
|
0.9 |
2.8 |
4.3 |
7.2 |
8.2 |
|
ACR FM criteria (+) (%) |
0.0 |
0.0 |
0.0 |
100.0 |
100.0 |
|
PSD (0-31) |
1.7 |
5.4 |
9.3 |
15.4 |
24.5 |
|
Widespread pain (%) |
0.8 |
18.0 |
57.5 |
87.8 |
100.0 |
Disclosure:
F. Wolfe,
None;
B. T. Walitt,
None;
J. Rasker,
None;
R. S. Katz,
None;
W. Häuser,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/polysymptomatic-distress-categories-for-clinical-and-research-use/