Session Information
Date: Tuesday, November 15, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: High ACR responses in placebo group have been frequently observed in recent RA trials, most notably, in patients with inadequate response (IR) to methotrexate (MTX) or other disease modifying anti-rheumatic drugs (DMARDs). The factors that contribute to high placebo responses in RA trials were investigated.
Methods: Total 10 ph2 and 3 RA trials in a database were pooled for meta-analysis (981 placebo subjects). All were randomized, double-blind, placebo-controlled trials in active RA MTX/DMARD-IR (N=9) or TNF-IR (N=1) populations in combination with stable doses of MTX (N=8) and/or other DMARDs (N=2). Baseline demographic and disease characteristics of participants were similar across these trials. Placebo response in each ACR component was summarized. Univariate logistic regression was used to identify patient baseline risk factors for placebo response after adjusting for study and time point.
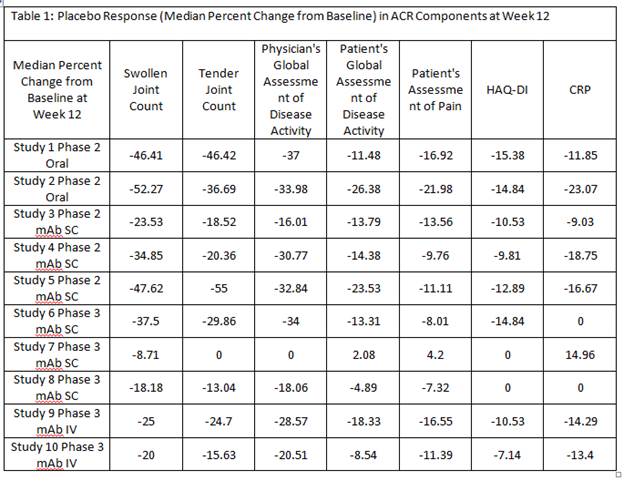
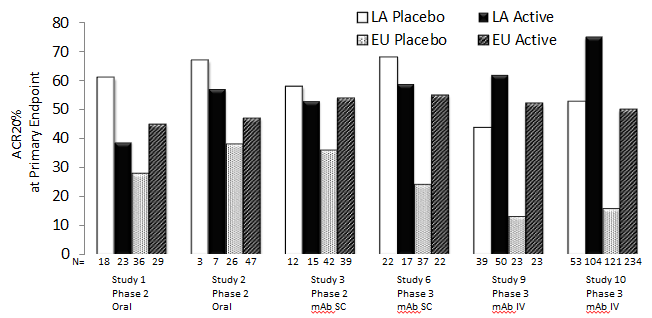
Results: In each of the 10 RA trials, at Week 12, median percent improvement from baseline in placebo group was consistently higher in physician/site staff assessments, ie swollen/tender joint counts, and physician’s global assessment of disease activity, compared to patient’s assessment of pain, global assessment of disease activity and function (HAQ-DI). Decrease in CRP in response to placebo was also observed (Table 1) however, often with more variability compared to other ACR components (data not shown). Patient baseline characteristics significantly predicting ACR20 placebo response are summarized in Table 2. A higher ACR Placebo response was observed in Latin America compared to Europe in 6 out of 7 RA trials that included Latin American sites (Figure 1).
Conclusion: The assessments performed by investigator or site staff are more sensitive to placebo response compared to the patient reported outcomes. Multiple patient baseline risk factors for ACR20 placebo response were identified and further investigation using multivariate model is warranted to delineate the independent predictors for high placebo response in RA trials. The Latin American region showed a consistently high placebo response across RA trials which often led to diminishing treatment effect detectable in this region.
Figure 1: High ACR20 Placebo Response in the Latin American Region Observed in 6 RA Trials
To cite this abstract in AMA style:
Xu X, Dong B, Hsu CH, Hu C, Lei C, Song J, Lu J, Beutler A. Physician/Site Staff Assessments Contribute to High Placebo Response in Rheumatoid Arthritis Clinical Trials [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/physiciansite-staff-assessments-contribute-to-high-placebo-response-in-rheumatoid-arthritis-clinical-trials/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/physiciansite-staff-assessments-contribute-to-high-placebo-response-in-rheumatoid-arthritis-clinical-trials/