Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Current quality of care guidelines recommend that all patients with Rheumatoid Arthritis (RA) be treated with biologic and/or non-biologic (nb) DMARDs. However, some RA patients are not treated with these agents for a variety of reasons, which may impact perceptions of the quality of care their physicians are providing.
To estimate physician-level variability on the proportion of RA patients who receive no biologic or nbDMARDs over a sustained period of time in a large U.S. Registry.
Methods:
Data from the Consortium of Rheumatology Researchers of North America (CORRONA) was used to identify rheumatologist-diagnosed RA patients. Patients were characterized as being on RA treatment (biologics and/or nbDMARDs, irrespective of glucocorticoids/NSAID/narcotics) based upon their most 3 consecutive visits in the registry. They were categorized as consistently on no treatment (0 of 3 visits), consistently on treatment (3 of 3 visits), and inconsistently on treatment (1-2 of 3 visits). Alternating logistic regression was used to evaluate physician-level clustering, controlling for patient-level factors including patient age, sex, RA disease duration, disease activity measured by CDAI, disability (by mHAQ), and comorbidities.
Results:
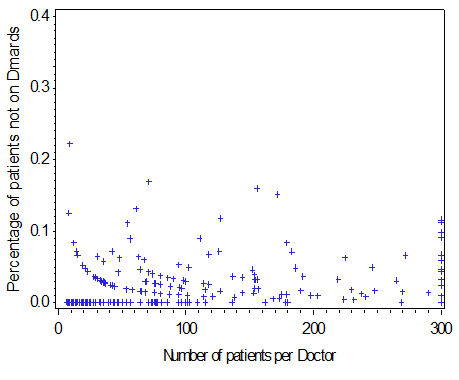
A total of 361 CORRONA physicians and 22,889 of their RA patients contributed to the analysis (median [IQR] number of RA patients per physician 19 [4, 78]). Using data from the 3 most recent CORRONA visits, 3.5 % of patients were consistently on no treatment, 84.7% consistently on treatment, and 11.8% inconsistently on treatment. The proportion of physicians’ patients on no RA treatment is shown (Figure) and ranged from 0 – 20%. For patients consistently on no RA treatment (n = 805), 31.4% were in remission, 37.1% were in low disease activity, and 31.4% were in moderate or high disease activity at the most recent CORRONA visit. After controlling for patient factors, physician clustering was independently associated with RA patients being on no RA treatment (adjusted OR = 1.81, 95% CI 1.17 – 2.80). Ongoing work is evaluating other potential reasons why patients may be untreated.
Conclusion:
A modest proportion of RA patients are treated with no biologics or DMARDs over a sustained period of time. Even after controlling for RA-related and other patient characteristics, meaningful variability in the proportion of patients on no RA treatment was observed between physicians, suggesting the possibility of inappropriate variation in quality of care. Further efforts to explain and/or ameliorate quality gaps in RA appear warranted and have implications on reporting systems that judge rheumatologists’ quality of care. Except in the circumstance where patients are in clinical remission, codifying other evidence-based reasons why offering no RA treatment is appropriate is likely to be useful.
Figure: Proportion of Rheumatologists’ RA patients consistently on no treatment
Disclosure:
D. A. Pappas,
Novartis ,
9;
L. Chen,
None;
L. R. Harrold,
Corrona,
5,
Takeda,
2;
G. W. Reed,
Corrona, Inc,
3;
J. M. Kremer,
Pfizer, Lilly,
2,
Pfizer, Lilly, Vertex,
5;
J. D. Greenberg,
None;
J. R. Curtis,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abb Vie,
2,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abb Vie,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/physician-variability-in-rheumatoid-patients-not-receiving-biologics-or-non-biologic-dmards-implications-for-quality-reporting/