Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: It is unclear if the elevated risks of adverse events in rheumatoid arthritis (RA) patients are due to use of biologics or DMARDs, glucocorticoids (GCs), or features associated with the disease itself. The selection of RA medications is potentially confounded due to disease activity and severity (i.e. channeling). Physician proclivities to use specific medications have been shown in prior analyses to be partially independent of patient factors and may represent ‘natural variability’ to reduce confounding in observational analyses. Therefore, we evaluated variability in physicians’ proclivity to use oral GCs among older RA patients.
Methods: Using 2009 Medicare data, we identified patients who had at least two RA diagnosis codes from rheumatologists. We classified patients as oral GC users if they had at least one prescription for >= 30 days’ supply. Rheumatologists were identified in the data using NPI numbers.
We calculated proclivity to use GCs for each rheumatologist as a proportion, computed as the number of their RA patients who used GCs divided by total number of all their RA patients, and categorized each rheumatologist into one of 4 groups based on quartiles. For each rheumatologist, this proportion was plotted against the number of RA patients in their practice.
The analysis was repeated in the subgroup of patients who also used biologics in 2009. Physician variability in steroid use was examined based upon inter-quartile ranges of the proportion of their patients using GCs, as well as measures of central tendency (i.e. skewness and kurtosis). Weighted Kappa statistics evaluated whether assignment of physicians’ steroid proclivity to quartiles was consistent between the main analysis and the subgroup analysis.
Results: We identified 313,108 RA patients (27,474 of whom used biologics) treated by 4,003 rheumatologists in 2009 Medicare data. Overall, 81,422 (26%) pts were oral GC users; 12,306 (44.8%) in the biologic subgroup used GCs.
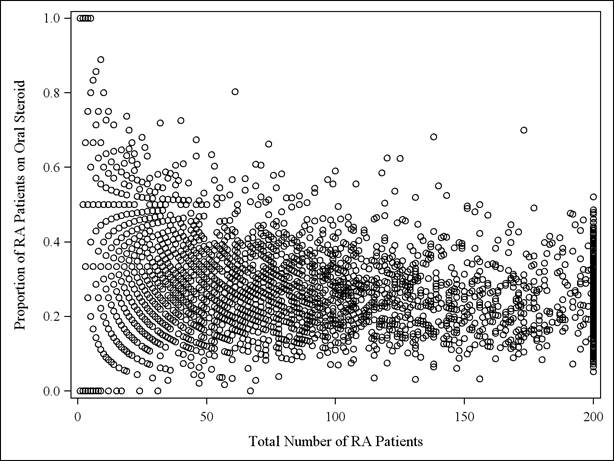
The proportion of patients using oral GCs was plotted for each U.S. rheumatologist (Figure) and demonstrated substantial variability between physicians. After restricting to patients on biologics, there was greater physician variability in use of GCS for their patients than in the main analysis (not shown). The weighted kappa statistic comparing physician’s proclivity to use GCs using all their RA patients vs. proclivity to use GCs in the biologic-using subgroup was 0.32 (95% confidence limits: 0.30-0.35) indicating low to moderate agreement.
Conclusion: The frequency with which rheumatologists’ prescribe oral GCs for older RA patients was highly variable and was even greater for patients using biologics. Further work is ongoing to examine whether this approach might be effect to reduce unmeasured or residual confounding associated with glucocorticoid use.
Figure: Proportion of rheumatologist’s RA patients on glucocorticoids per practice in 2009
Disclosure:
H. Yun,
None;
F. Xie,
None;
E. S. Delzell,
Amgen,
2;
L. Chen,
None;
E. Levitan,
Amgen,
2;
J. Lewis,
Pfizer, Prometheus, Lilly, Shire, Nestle, Janssen, AstraZeneca, Amgen,
5,
Centocor, Shire, Takeda,
2;
K. G. Saag,
Ardea; Regeneron; Savient; Takeda,
5,
Ardea; Regeneron; Savient: Takeda,
2;
T. Beukelman,
Genentech and Biogen IDEC Inc.,
5,
Novartis Pharmaceutical Corporation,
5,
Pfizer Inc,
2;
K. L. Winthrop,
Pfizer Inc,
2,
Genentech Inc., Pfizer, UCB, Regeneron,
5;
J. Baddley,
BMS,
2,
Pfizer Inc,
2;
J. R. Curtis,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abb Vie,
2,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abb Vie,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/physician-proclivity-to-use-oral-glucocorticoids-among-rheumatoid-arthritis-patients/