Session Information
Date: Tuesday, October 23, 2018
Title: Systemic Sclerosis and Related Disorders – Clinical Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic sclerosis (SSc) patients have reduced physical function, exercise capacity and health related quality of life (HRQOL). The objective of this study was to assess the feasibility and test-retest reliability of physical activity trackers as a measure of physical activity in patients with SSc.
Methods: SSc subjects, based on 2013 ACR/EULAR classification criteria, 18 to 79 years old without large joint contractures or use of mobility devices, and with access to a smartphone or computer were eligible; if subjects had pulmonary hypertension (PH) and/or interstitial lung disease (ILD), medications were stable for 3 months prior to enrollment. Subjects received a Fitbit Zip® physical activity tracker to wear daily during awake hours and performed their everyday activities; no step goals were provided. Patient reported outcomes (PROs) were provided at baseline and follow up visit.
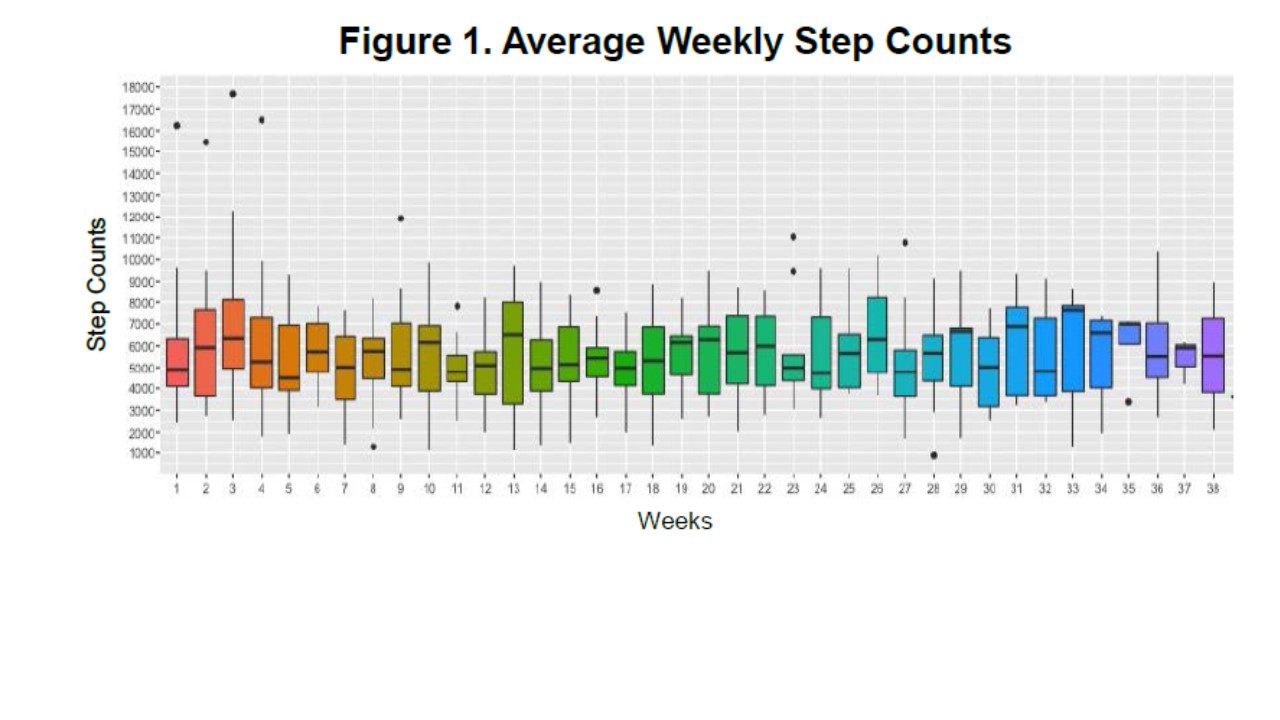
Results: Twenty subjects have been recruited; 75% were female, 90% were Caucasian, 50% had diffuse cutaneous SSc, 40% had limited cutaneous systemic sclerosis, 10% had sine scleroderma, 35% had ILD, 10% had PH, mean (SD) age was 49 (11.7) years old and SSc disease duration was 5.3 (3.6) years. PROMIS-29 mean (SD) values at baseline (N=19) were as follows: physical function 47.2 (7.9), social roles 53.3 (8.4), anxiety 49.5 (9.7), depression, 47.3 (8.8), fatigue 50.0 (9.4), sleep disturbance 49.7 (7.3), pain interference 51.7 (7.7), and pain intensity 2.6 (2.4). Two subjects withdrew after enrollment and did not use the tracker. Mean (SD) study duration for the remaining 18 subjects was 177.7 (89.2) days and mean (SD) daily step counts during the first week were 5710.8 (3212.4) steps (Figure 1). Mixed effects model to assess test-retest reliability of weekly step counts for each subject indicated step counts were stable over time (Figure 2). PROMIS-29, UCSD SOBQ, SHAQ, and mMRC PROs did not indicate a statistically significant difference from baseline to follow up (data not shown, p>0.05). On comparison of subjects without ILD and/or PH (N=13) to those with ILD and/or PH (N=7), mean (SD) daily steps counts were numerically higher in those without ILD and/or PH (6523 vs 4925, p = 0.29) but there were statistically significant differences for PROMIS-29 physical function (50.18 vs 41.5, p=0.047), mMRC grade (0.33 vs 1.33, p=0.018), and VAS breathing problems (0.5 vs 2.17, p=0.035).
Conclusion: Physical activity trackers show acceptable feasibility and test-retest reliability for use in SSc. Future studies will evaluate change in HRQOL with use of step count goal interventions.
To cite this abstract in AMA style:
Young A, Jackson EA, Khanna D. Physical Activity Trackers Work Well As a Monitor of Physical Activity in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/physical-activity-trackers-work-well-as-a-monitor-of-physical-activity-in-systemic-sclerosis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/physical-activity-trackers-work-well-as-a-monitor-of-physical-activity-in-systemic-sclerosis/