Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Ianalumab is a novel defucosylated human IgG1 mAb targeting the receptor for B cell Activating Factor belonging to the TNF Family (BAFF-R) providing potent B cell depletion by enhanced antibody-dependent cellular cytotoxicity along with BAFF:BAFF-R signaling blockade.We report safety and efficacy of ianalumab in patients with active SLE.
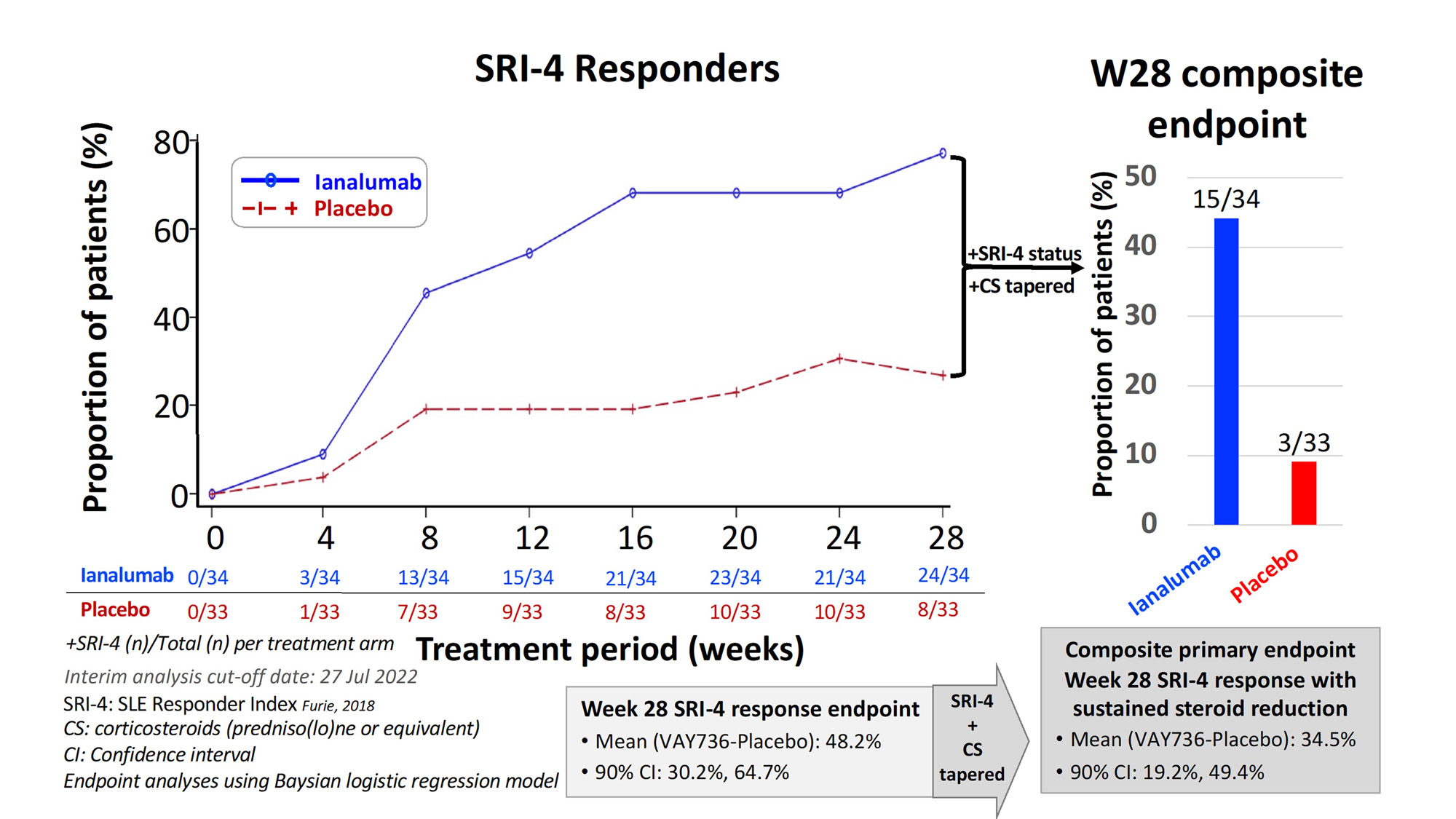
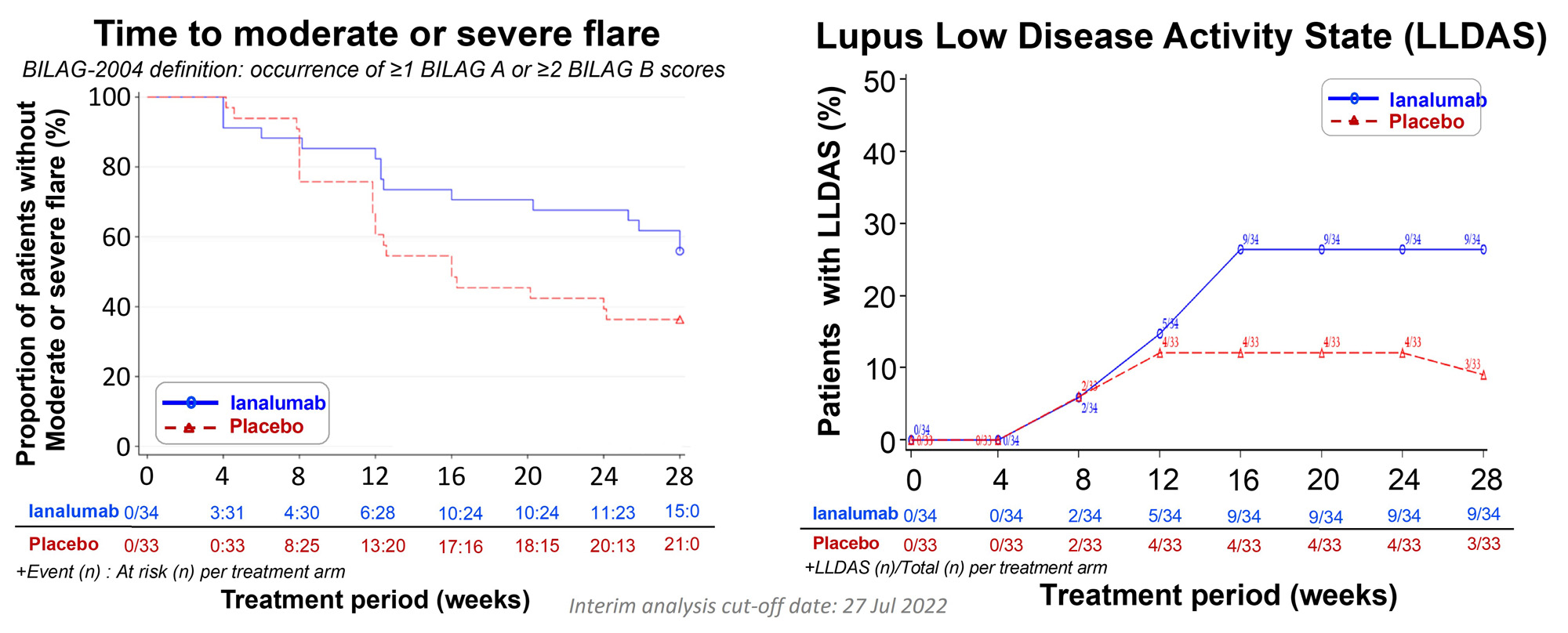
Methods: This multi-center randomized, parallel group, double-blind placebo-controlled umbrella trial (NCT03656562) enrolled patients (19 Dec 2018 to 31 Jan 2022) having ANA ≥1:80 and meeting ≥4 of 11 ACR 1997 SLE classification criteria, with SLEDAI-2K score ≥6 and BILAG-2004 ≥1 A or ≥2 B scores that included activity in either mucocutaneous and/or musculoskeletal domains. This report is limited to interim analysis results for the fully enrolled ianalumab treatment cohort (active n=34; placebo n=33) completing 28-week blinded treatment period (monthly s.c. injection ianalumab 300 mg or placebo), with measured outcomes at baseline and weeks (w) 4, 8, 12, 16, 24 & 28. Primary w28 outcome was proportion patients meeting composite endpoint requirements consisting of those achieving SLE-Responder Index (SRI)-4 who also tapered predniso(lo)ne to ≤5 mg/d or ≤ baseline dose, whichever was lower, by w16 and kept within that range to w28.Secondary/exploratory outcomes included safety/tolerability, incidence BILAG-2004 moderate or severe flares (≥1 A or ≥2 B), proportion patients achieving Lupus Low Disease Activity State (LLDAS), patient and physician global assessments, and laboratory markers of B cell-associated autoimmune activity.
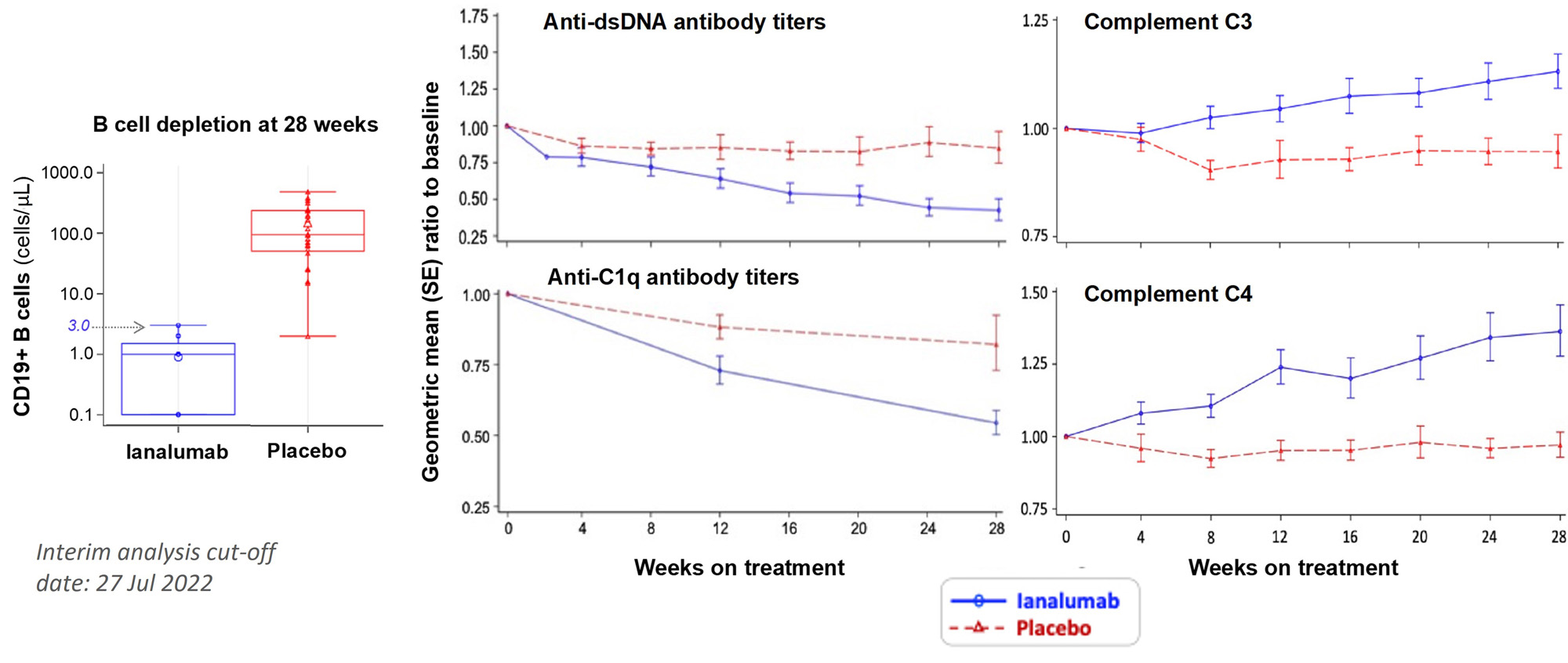
Results: VAY736 was safe and well-tolerated with no drug-related SAE or dropouts, and one pandemic-related discontinuation in placebo arm. Baseline median(range) values for ianalumab and placebo arms, respectively, were: SLEDAI-2Kscore 10 (6-32) and10 (4-18), and predniso(lo)ne 10 mg (0-30) and 10 mg (0-27.5). Marked depletion of circulating CD19+ B cells was consistently achieved in all ianalumab-treated patients: w28 median 1 cell/uL; range 0-3 (Fig. 3). The mean w28 SRI-4 treatment effect of ianalumab (n=24) over placebo (n=8) was 48.2% (Fig. 1), and that also met the composite primary endpoint was 34.5% (ianalumab n=15; placebo n=3). Reduced incidence of moderate or severe flare was noted for patients treated with ianalumab 44% (n=15) vs placebo 64% (n=21). Benefits of ianalumab over placebo were observed for time-to-moderate or -severe flare and for achieving w28 LLDAS (Fig. 2). Therapeutic responses were also observed for ianalumab- vs placebo-treated subjects on laboratory markers of autoimmune activity (Fig. 3).
Conclusion: Potent B cell depletion was consistently achieved in ianalumab-treated SLE patients that was well tolerated, achieving the primary endpoint of SRI-4 response with sustained steroid reduction, along with substantial treatment benefits on overall SRI-4 response, LLDAS and reductions in moderate and severe flares, and on laboratory markers of autoimmune activity. These positive phase 2 study results support ongoing phase 3 development in lupus of the dual mechanisms of action of ianalumab (SIRIUS-SLE 1 & 2, and SIRIUS-LN).
To cite this abstract in AMA style:
Shen N, Ignatenko S, Gordienko A, Cortés Hernández J, Agmon-Levin N, Narongroeknawin P, Romanowska -Prochnicka K, Ciferska H, Kodera M, Wei J, Leszczynski P, Lan J, Mysler E, Wojciechowski R, Tarr T, Vishneva E, Chen Y, Kaneko Y, Finzel S, Hoi A, Koolvisoot A, Lee S, Dai L, Kaneko H, Rojkovich B, Sun L, Zotkin E, Viallard J, Katayama M, Magallares-Lopez B, Sengupta T, Sips C, J Oliver S. Phase 2 Safety and Efficacy of Subcutaneous (s.c.) Dose Ianalumab (VAY736; Anti-BAFFR mAb) Administered Monthly over 28 Weeks in Patients with Systemic Lupus Erythematosus (SLE) of Moderate-to-Severe Activity [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/phase-2-safety-and-efficacy-of-subcutaneous-s-c-dose-ianalumab-vay736-anti-baffr-mab-administered-monthly-over-28-weeks-in-patients-with-systemic-lupus-erythematosus-sle-of-moderate-to-severe/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phase-2-safety-and-efficacy-of-subcutaneous-s-c-dose-ianalumab-vay736-anti-baffr-mab-administered-monthly-over-28-weeks-in-patients-with-systemic-lupus-erythematosus-sle-of-moderate-to-severe/