Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Tocilizumab is a biologic anti-interleukin-6 receptor monoclonal immunoglobin G1 antibody indicated for treating inflammatory diseases, including RA, and cytokine release syndrome. MSB11456 is a proposed biosimilar to US-licensed tocilizumab and EU-approved tocilizumab. This randomized, double-blind, parallel-group single-dose study (EudraCT Number 2019-003484-22) compared the pharmacokinetic (PK), safety, tolerability, and immunogenicity profiles of intravenous (IV) MSB11456 versus US-licensed tocilizumab in healthy adult volunteers. The primary objective was to demonstrate the PK equivalence of MSB11456 with US-licensed tocilizumab after a single IV infusion, following applicable guidelines from the European Medicines Agency and US Food and Drug Administration.
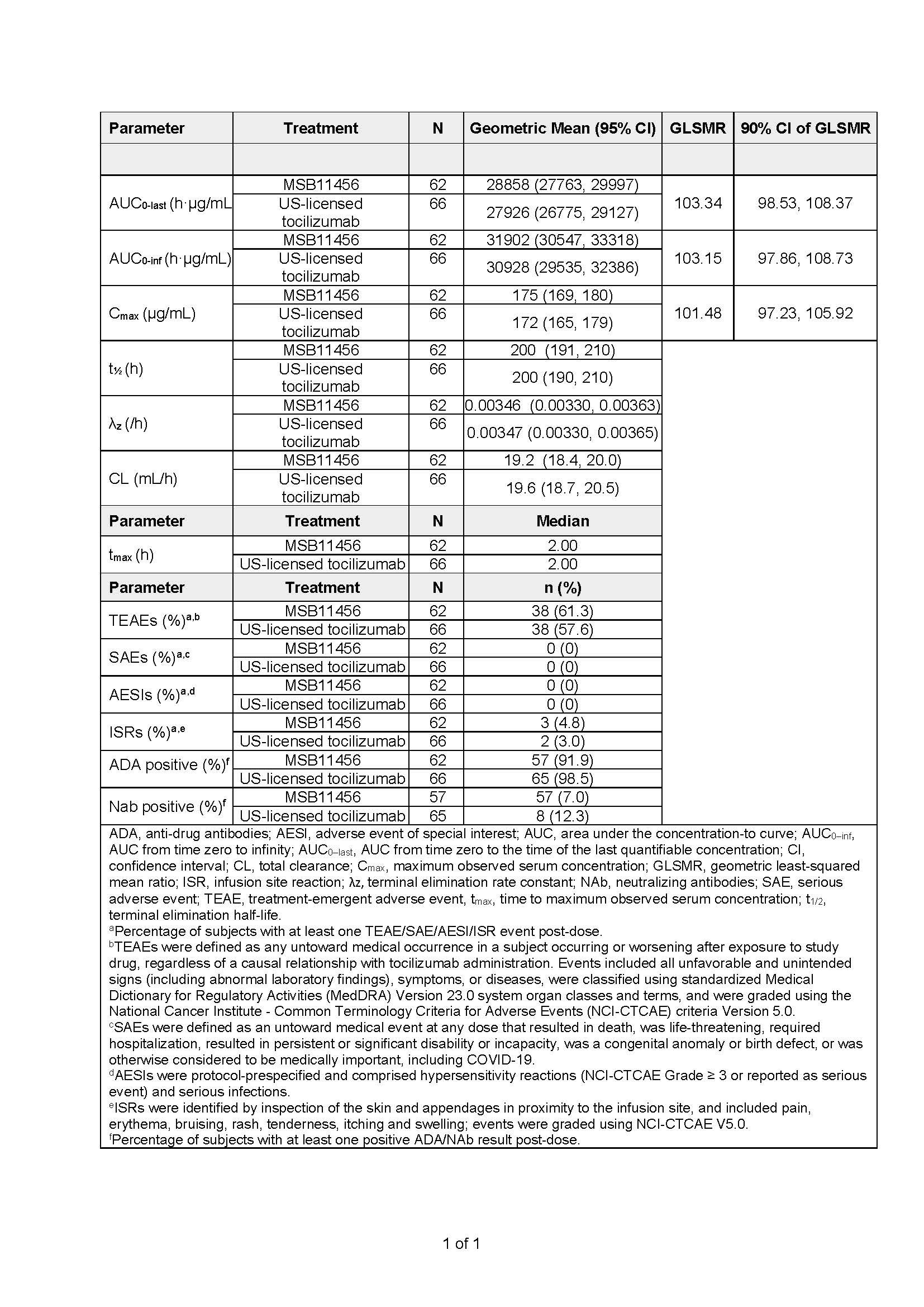
Methods: Healthy adults were randomized to receive MSB11456 or US-licensed tocilizumab as a single 1-hour 8 mg/kg IV infusion. Blood samples for PK and immunogenicity analyses were collected pre-dose and at scheduled time points up to day 48 post-dose. To demonstrate PK equivalence, the primary endpoint was the area under the concentration-time curve (AUC) from time zero to the last quantifiable concentration (AUC0-last). Secondary endpoints included maximum observed concentration (Cmax), AUC from time zero to infinity (AUC0-inf), additional PK and safety parameters, antidrug antibody (ADA), and neutralizing antibody (NAb) incidence (Table 1). PK equivalence between the two treatments was demonstrated if the 90% confidence interval (CI) for the geometric mean ratio (GMR) for geometric least squares mean (GLSMR) AUC0-last, was entirely contained within the 80.00% to 125.00% equivalence limits. The primary analysis was performed on natural logarithm (ln) transformed parameters using an analysis of variance model with treatment as a fixed effect.
Results: 130 subjects were randomized; 2 subjects were withdrawn prior to dosing due to adverse events. A total of 128 subjects received tocilizumab, of whom 62 received MSB11456 and 66 received US-licensed tocilizumab. Demographic characteristics of both treatment groups were similar. Primary PK endpoint analysis demonstrated equivalence between MSB11456 and US-licensed tocilizumab with 90% CI for the GLSMR AUC0-last entirely contained within the 80.00% to 125.00% equivalence limits (GLSMR [90% CI] 103.34 [98.53, 108.37]). 90% CI for the GMR for the secondary PK parameters AUC0-inf (103.15 [97.86, 108.73]), and Cmax (101.48 [97.23, 105.92]) supported the conclusion of PK equivalence. Safety, tolerability, and immunogenicity were similar between groups (Table 1).
Conclusion: After a single IV infusion of 8 mg/kg in healthy subjects, PK equivalence was demonstrated between MSB11456 and US-licensed tocilizumab for the primary and secondary PK endpoints, with similar safety, tolerability, and immunogenicity results for each product. This study supports IV MSB11456 as a proposed biosimilar to US-licensed tocilizumab.
To cite this abstract in AMA style:
Tomaszewska-Kiecana M, Ullmann M, Vincent E, Petit-frere C, Monnet J, Illes A. Pharmacokinetics, Safety, Tolerability, and Immunogenicity of a Proposed Tocilizumab Biosimilar (MSB11456) versus US‑licensed Tocilizumab: Results of a Randomized, Double-blind, Parallel‑group, Single‑intravenous Dose Study in Healthy Adults (APTURA II) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/pharmacokinetics-safety-tolerability-and-immunogenicity-of-a-proposed-tocilizumab-biosimilar-msb11456-versus-us%e2%80%91licensed-tocilizumab-results-of-a-randomized-double-blind-parallel/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-safety-tolerability-and-immunogenicity-of-a-proposed-tocilizumab-biosimilar-msb11456-versus-us%e2%80%91licensed-tocilizumab-results-of-a-randomized-double-blind-parallel/