Session Information
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment: Treatment and Management Studies
Session Type: Abstract Submissions (ACR)
Background/Purpose : B-cell activating factor (BAFF) promotes B-cell survival and maturation, and increased serum levels are associated with autoimmune disease and disease activity in systemic lupus erythematosus (SLE). Tabalumab is a human anti-BAFF monoclonal antibody that neutralizes both soluble and membrane-bound BAFF. The objectives of 2 Phase 1 studies were to evaluate the pharmacokinetics (PK), safety, and biological activity of tabalumab administered intravenously (IV) or subcutaneously (SC) in subjects with rheumatoid arthritis (RA) or SLE.
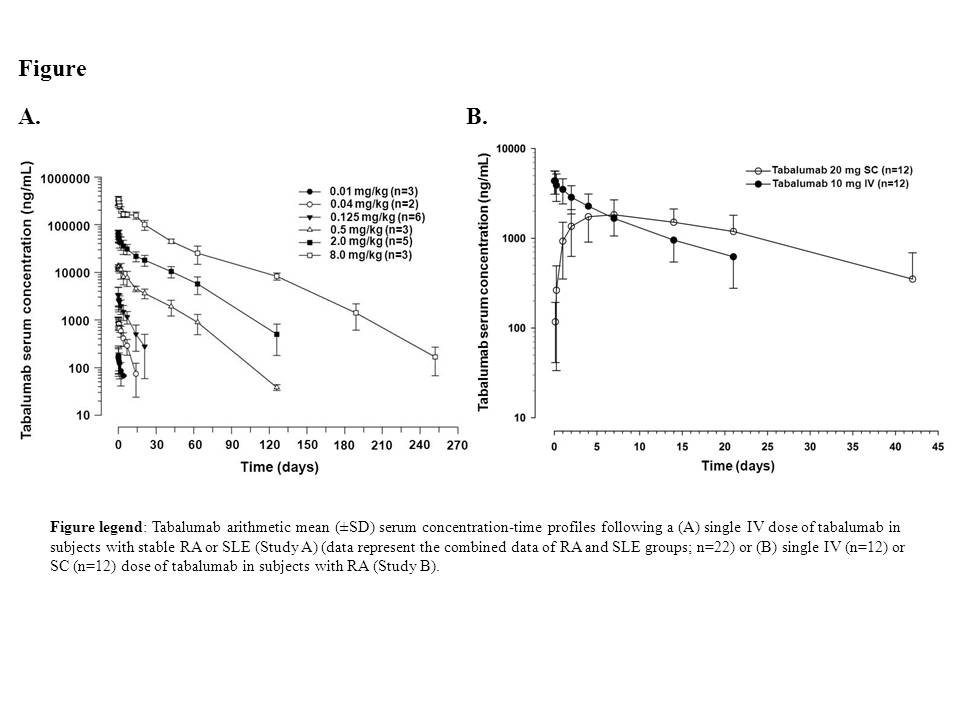
Methods: In Study A, subjects with stable RA (n=23) received a single dose of tabalumab ranging from 0.01 to 8.0 mg/kg (0.01, 0.04, 0.125, 0.5, 2.0, and 8.0 mg/kg) or placebo, and subjects with stable SLE (n=6) received 1 of 2 tabalumab doses (0.125 or 2.0 mg/kg) or placebo by IV infusion. In Study B, subjects with RA received a single tabalumab dose SC (20 mg) (n=12) or IV (10 mg infused over 30 min) (n=12). Serum tabalumab and peripheral CD20+ B-lymphocyte levels were evaluated for 36 weeks in Study A and 12 weeks in Study B with optional follow up to monitor B-lymphocyte recovery, if required. Safety was assessed throughout both studies.
Results: Tabalumab PK were nonlinear across the 0.01 to 8.0 mg/kg dose range (Figure A), with a slower clearance (CL) and longer half-life (t1/2) at higher doses. The CL decreased from 2.9 to 0.1 L/day over the dose range, resulting in greater than dose-proportional increases in exposure. The terminal t1/2 increased from 1.6 to 25 days over the dose range. PK parameters were similar between RA and SLE subjects. SC dosing had a slow absorption phase with time to maximal concentration (tmax) of approximately 5.5 days. The estimated bioavailability (F) for the 20-mg SC dose was 62%. Single-dose tabalumab was well tolerated, and the majority of treatment-emergent adverse events (TEAEs) were mild to moderate in severity in both studies. The rate of TEAEs was similar for IV and SC groups in Study B. TEAEs considered related to study drug included headache (n=1), back pain (n=1), dry mouth (n=1), dysgeusia (n=1) dysphagia (n=1), and nausea (n=3) in study A, and injection-site pain (n=1) and flushing (n=1) in the 20-mg SC group in Study B. In general, a tabalumab-dependent transient increase from baseline in CD20+ B lymphocytes followed by a progressive decrease below baseline levels was observed in both studies, with the decrease being significant (P<0.05; overall F-test) for the 2-mg/kg and 8-mg/kg doses. No increases in anti-tabalumab antibodies were detected post-treatment.
Conclusion: A single tabalumab dose administered IV or SC was well tolerated and had non-linear CL over the dose range investigated in subjects with RA and SLE. The non-linearity likely reflects target-mediated CL due to binding to BAFF. Tabalumab showed biological activity based on changes in peripheral CD20+ lymphocyte numbers in both subjects with RA and SLE.
Disclosure:
J. Witcher,
Eli Lilly and Company,
3,
Eli Lilly and Company,
1;
R. Hansen,
Eli Lilly and Company,
3,
Eli Lilly and Company,
1;
L. Hu,
Eli Lilly and Company,
3,
Eli Lilly and Company,
1;
D. Radtke,
Eli Lilly and Company,
1,
Eli Lilly and Company,
3;
J. Voelker,
Eli Lilly and Company,
1,
Eli Lilly and Company,
3;
J. McColm,
Eli Lilly and Company,
1,
Eli Lilly and Company,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-safety-and-biological-activity-of-intravenously-or-subcutaneously-administered-tabalumab-in-subjects-with-rheumatoid-arthritis-or-systemic-lupus-erythematosus/