Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Monthly (q4w) intravenously (IV) administered belimumab 10 mg/kg is approved for the treatment of adults with active, autoantibody-positive SLE receiving standard therapy. The present analysis was conducted to characterize the exposure and exposure-response of belimumab administered subcutaneously (SC) in adult SLE patients.
Methods: Belimumab PK (n=554) and clinical response data from the SC SLE Phase 3 trial (BEL112341/NCT01484496) combined with serially sampled PK data in healthy volunteers from two Phase 1 trials (n=134; BEL114448/NCT01583530, BEL116119/NCT01516450) were analyzed with a population PK/PD (popPK/PD) approach. Following popPK model development, typical PK profiles were simulated using population parameters from this analysis and the IV popPK. A logistic regression model for efficacy response (SRI; SLE Responder Index) was developed to characterize dependencies of SRI response to individual exposures and other patient characteristics.
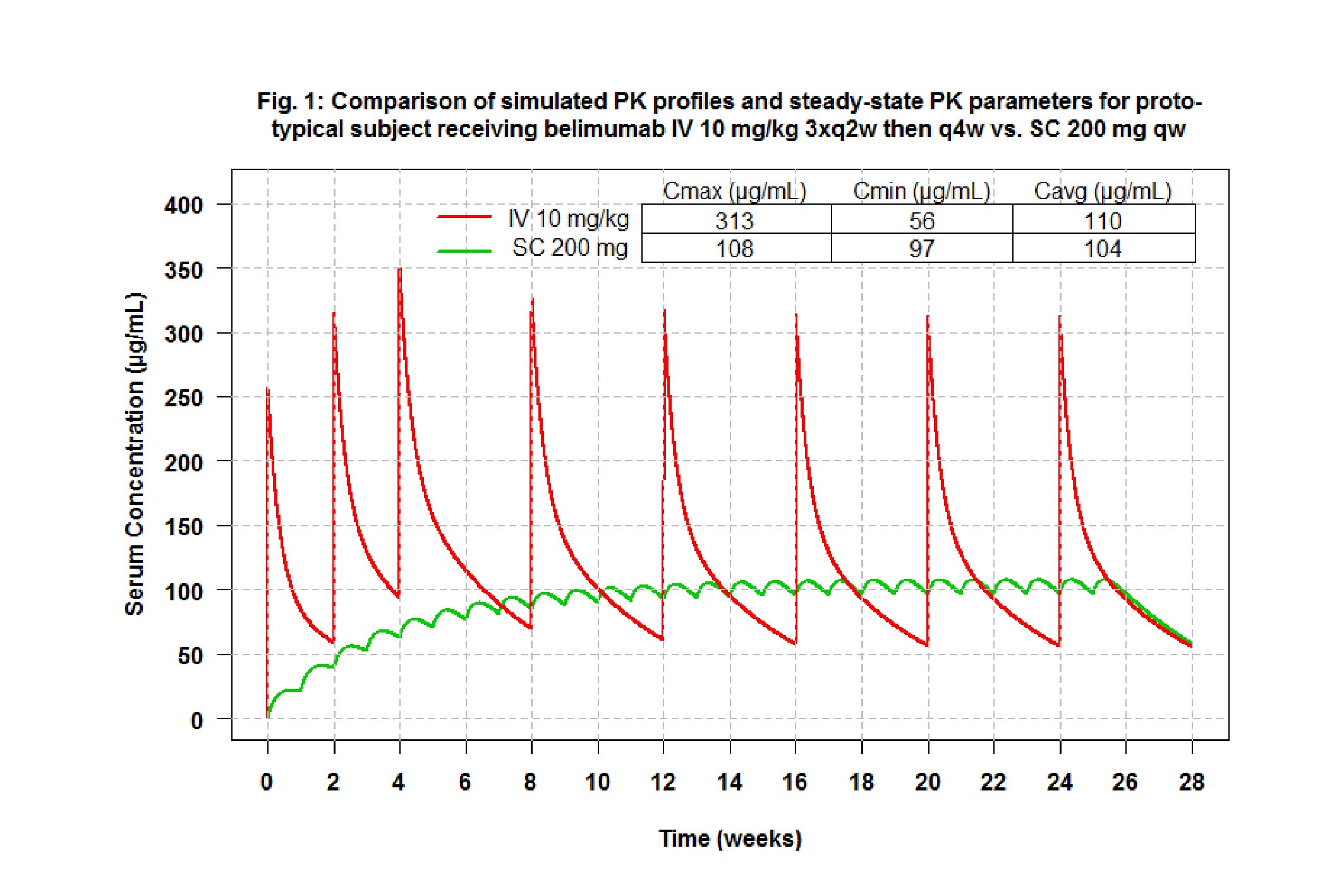
Results: The PK of belimumab administered SC was best described by a linear 2-compartment model with first order absorption and absorption lag time with a terminal half-life of 18.3 days. Body weight, BMI, albumin and IgG had statistically significant effects on the PK parameters, albeit with a minor effect size compared to random exposure variability. Simulations with PK parameters from this and the IV popPK analysis, demonstrated that the weekly 200 mg SC regimen results in steady-state Cavg equivalent to the 10 mg/kg IV every 4 weeks regimen (Fig.1). In the final SRI logistic regression model exposure (Cavg) was not statistically significant (α=0.05); only baseline disease activity (SELENA-SLEDAI), proteinuria (>0.5 g/day), African-American and American-Indian/Alaskan-Native race categories were identified as significant predictors of responder status.
Conclusion: 200 mg SC qw dosing in SLE patients resulted in steady-state belimumab Cavg comparable to the Cavg for 10 mg/kg IV q4w dosing, the approved belimumab IV dosing regimen. The popPK/PD analysis showed that exposure did not have a statistically significant impact on the SRI response. These results confirm that 200 mg qw belimumab is appropriate for subcutaneous administration to SLE patients and that no dose adjustment is required for adult patients to maintain efficacy and safety demonstrated in BLISS-SC. Disclosure: GSK funded this study.
To cite this abstract in AMA style:
Struemper H, Thapar M, Gordon D, Roth D. Pharmacokinetics and Exposure-Response Relationship of Belimumab Administered Subcutaneously to SLE Patients [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/pharmacokinetics-and-exposure-response-relationship-of-belimumab-administered-subcutaneously-to-sle-patients/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-and-exposure-response-relationship-of-belimumab-administered-subcutaneously-to-sle-patients/