Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Mouse collagen induced arthritis (CIA) is the most commonly used preclinical model to screen new drug candidates for inflammatory arthritis. The conventional read out of this model are clinical score and histopathology. These read outs have several limitations including (i) longitudinal studies using the same mouse cannot be performed; (ii) clinical and histopathological scores are subjected to observer bias; (iii) in vivo cellular events cannot be captured in its native environment. Thus, an in vivo drug screening tool is the unmet need of the day. Herein, we validated 18F-FDG PET as an in vivo drug screening tool for new anti-inflammatory drugs using the mouse CIA model.
Methods: Animal handling was performed in accordance with the approved UC Davis IACUC protocol. Arthritis was induced using bovine type II collagen in 8-12 week old male DBA/1J mice (n=20), out of which 15 mice showed clinical signs of arthritis on day 28. After the disease progressed, on the day 42 to identify the pre-treatment histopathology 5 mice were sacrificed. In the remaining 10 mice, 5 mice received I.P 300 μg of anti-mouse TNF-α antibody (CNTO5048, Janssen Biotech, PA, USA) every alternate day for next 10 days, and 5 mice remained untreated (negative control). Mice were scored clinically and had 18F-FDG PET scan on day 42 and 52. Histological score (HS) of the joint tissues were performed in all the mice.
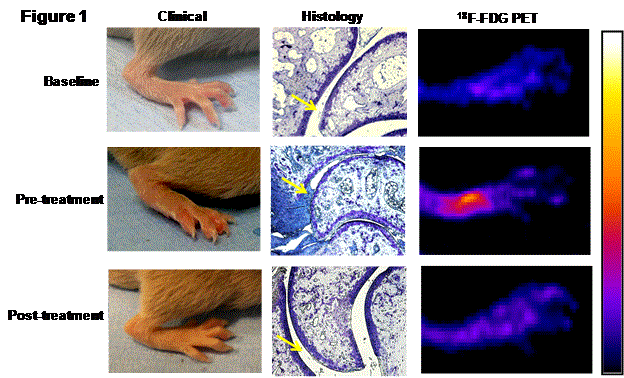
Results: The pre-treatment mean clinical score (CS), histopathological score (HS), and 18F-FDG uptake were 3.8±1.1, 9±1 and 340±22 kBq/cc (Mean±SD), respectively. In the untreated group, the CS, HS and 18F-FDG uptake progressed on day 52 to 5.4±1.2 (p<0.05), 13.4±1.3 (p<0.05) and 480±32 KB1/cc (p<0.05), respectively. Anti-TNF therapy successfully arrested the disease progression as evident by CS and HS at day 52, 0.75±0.06 (p<0.01) and 2.5±1.1 (p<0.01), respectively compared to untreated group. 18F-FDG PET uptake in this group was also significantly decreased at day 52 to 200±12 KBq/cc (p<0.01) (Figure 1). The PET uptake significantly correlated with changes in CS (r2=0.74, p<0.01) and HS (r2=0.9, p<0.01). Among CS and HS, the correlation of 18F-FDG PET with HS was higher than that observed with CS.
Conclusion: Our observation strongly suggest that 18F-FDG PET scan can be considered as an in vivo preclinical drug screening tool for anti-inflammatory drugs of autoimmune arthritis. PET-CT imaging will reduce the number of animals required for a study and also the same animal can be studied at different stages of the disease, which will eventually reduce the effect of intra-species biological variations. The use of this in vivo imaging tool will allow longitudinal quantitative evaluation of the degree of joint inflammation in the same mouse.
Figure 1: 18F-FDG PET is an useful in vivo tool to assess therapeutic potential of antiinflammatory drugs (representative image).
Disclosure:
S. Raychaudhuri,
None;
A. Mitra,
None;
S. K. Raychaudhuri,
None;
A. Chaudhari,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pet-ct-imaging-of-joints-a-quantitative-tool-for-developing-novel-anti-inflammatory-drugs/