Session Information
Session Type: Abstract Submissions (ACR)
Title: Persistent Use of Biologic Therapies at Lower Than Recommended Dosing among Rheumatoid Arthritis Patients Enrolled in the US Medicare Program
Background/Purpose: Biologic therapy is associated with significant personal and societal costs. One possible strategy to reduce such cost is the use of reduced dose biologic whenever possible and the appropriateness of such an approach has been shown in a randomized clinical trial. The objective of the study is to examine the use of self-injectable biologics at reduced dose compared to the recommended dose in real world clinical practice.
Methods: We conducted a retrospective cohort study among patients with rheumatoid arthritis who were enrolled in the Medicare fee for service program with prescription drug coverage from 2006 to 2010. Eligible patients were required to have initiated either etanercept or adalimumab after a 12-month period with continuous coverage free of that agent. Follow-up started at treatment initiation and ended when a patient discontinued their biologic (> 90 day gap since end of days supply), switched to a different biologic, lost coverage, or died. For each patient, we calculated the number of days covered under each filled prescription assuming a constant rate of use (etanercept: 50 mg/week; adalimumab: 40 mg/2 weeks). We divided the follow-up time into 6-month intervals in which a patient remained under follow-up at the end of the interval; within each interval we calculated the proportion of days covered by the medications out of the total number of days. We defined reduced-dose biologic as having < 80% of the days covered and calculated the proportion of patients who continuously used reduced-dose biologic for 18 months, 24 months, and 30 months.
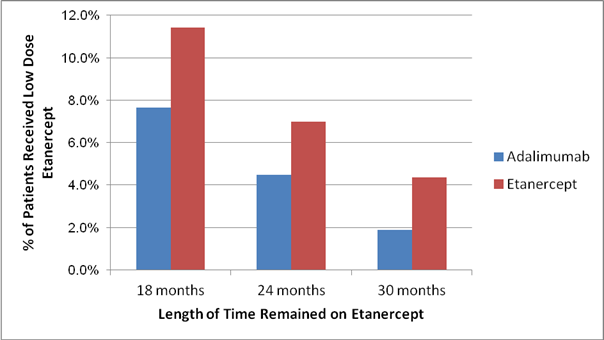
Results: We identified 3943 RA patients who were eligible to be included in this analysis, mean age 62 (standard deviation: 13); 83% were women; 2,541 initiated etanercept; and 2,389 initiated adalimumab. Among etanercept users, 11.4% of the patients received reduced-dose etanercept continuously for 18 months during their follow-up; 7.0% and 4.4% of the patient received reduced-dose etanercept continuously for at least 24 and 30 months, respectively. Among adalimumab users, the corresponding proportions were lower with 7.6%, 4.5%, and 1.9% of the persistent users received reduced-dose adalimumab for a continuous period of 18, 24, and 30 months. Data are also presented in the figure below.
Conclusion: In real-world clinical practice, up to 11% of RA patients received self-injectable biologics at a lower than the recommended dose for an extended period of time.
Figure Proportion of RA Patients who Continuously Used Reduced Dose Injectable Etanercept or Adalimumab
Disclosure:
J. Zhang,
Roche/Genentech,
2;
F. Xie,
None;
E. S. Delzell,
Amgen,
2;
H. Yun,
None;
J. Lewis,
Pfizer, Prometheus, Lilly, Shire, Nestle, Janssen, AstraZeneca, Amgen,
5,
Centocor, Shire, Takeda,
2;
K. Haynes,
None;
L. Chen,
None;
K. G. Saag,
Amgen,
2,
Eli Lilly and Company,
2,
Merck Pharmaceuticals,
2,
Amgen,
5,
Eli Lilly and Company,
5,
Merck Pharmaceuticals,
5;
J. R. Curtis,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, crescendo, AbbVie,
2,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, crescendo, AbbVie,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistent-use-of-biologic-therapies-at-lower-than-recommended-dosing-among-rheumatoid-arthritis-patients-enrolled-in-the-us-medicare-program/