Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Clinical evidence suggests concomitant
treatment with a biologic Disease-Modifying Antirheumatic
Drug (bDMARD) and a conventional synthetic DMARD (csDMARD), especially with methotrexate (MTX) has greater
efficacy than treatment with a bDMARD as monotheapy in patients with rheumatoid arthritis (RA). However,
not all patients are able to tolerate a csDMARD. Our
objective was to compare the persistence of a bDMARD
used as monotherapy, versus combination therapy in patients with active RA.
Methods:
Physician data were collected from the
Ontario Best Practices Research Initiative Rheumatoid Arthritis Registry (OBRI-
RA), a clinical registry of RA patients followed in routine care. Inclusion
criteria comprised of patients over age 18 years, active RA (defined as ³1 swollen joint) and started on their 1st bDMARD
within 30 days before registry enrolment, or started after enrolment. Combination
therapy was defined as treatment with a bDMARD plus
at least one csDMARD, while monotherapy was defined
as treatment with only a bDMARD.
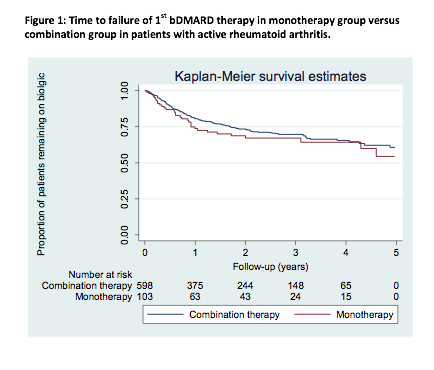
The primary outcome was persistence with 1st
bDMARD therapy, which was defined as the length of
time the patients continued to receive their first bDMARD
therapy. Persistence treatment was examined using Kaplan-Meier survival
analysis. Patients were censored at date of 1st bDMARD
stop, switch to another bDMARD or at date of last
follow-up, whichever came first.
Results:
Among 2591 RA patients, 701 patients started
their 1st bDMARD within 30 days before cohort
enrolment or after enrolment with the mean (standard deviation) of follow-up
1.9 (1.6) person-years. A total of 598 (85.3%) patients were on
combination therapy, and 103 (14.7%) patients were on monotherapy. At baseline,
there was a similar mean age, proportion of females between the two groups. A
TNFα inhibitor was the biologic used in 22.6% and 14.5% of the
monotherapy and combination group respectively.
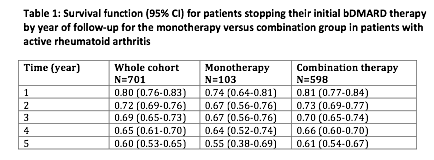
The mean time to failure of 1st bDMARD was 4.3 years (95%CI: 3.7-4.9) and 4.6 years (95%CI:
4.3-4.8) in the monotherapy and combination group respectively. At 12 months follow-up,
74% (95%CI: 64-81) in the monotherapy group and 81% (95%CI: 77 -84) in the combination group
remained on their first bDMARD (table 1).
Conclusion:
Our study demonstrates that a higher
proportion of patients on monotherapy failed therapy at 12 months, and the mean
time to treatment failure was shorter with monotherapy, but these results were
not statistically significant. Although combination therapy is recommended,
these real-world results suggest that patients who are unable/unwilling to
continue on a csDMARD, bDMARD
monotherapy can still provide an efficacious option.
To cite this abstract in AMA style:
Lau A, Movahedi M, Tatangelo M, Bombardier C. Persistence with Biologic Monotherapy in Comparison with Combination Therapy with Disease-Modifying Antirheumatic Drugs in Patients with Rheumatoid Arthritis; Results from a Rheumatoid Arthritis Cohort [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/persistence-with-biologic-monotherapy-in-comparison-with-combination-therapy-with-disease-modifying-antirheumatic-drugs-in-patients-with-rheumatoid-arthritis-results-from-a-rheumatoid-arthritis-cohor/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-with-biologic-monotherapy-in-comparison-with-combination-therapy-with-disease-modifying-antirheumatic-drugs-in-patients-with-rheumatoid-arthritis-results-from-a-rheumatoid-arthritis-cohor/