Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The treatment paradigm for psoriatic arthritis (PsA) has changed over the last two decades, with increasing numbers of therapy options that target a variety of inflammatory pathways. This analysis sought to examine treatment patterns among patients with PsA initiating either IL-17Ai or TNFi in order to understand patterns of persistence across biologics that utilize different mechanisms of action to treat PsA.
Methods: Adult patients newly initiating an IL-17Ai (ixekizumab or secukinumab) or TNFi biologic (adalimumab, certolizumab pegol, etanercept, golimumab, or infliximab) between April 1, 2016 and June 30, 2021 were identified in the MarketScan Commercial and Medicare Database. The first biologic claim served as the index date and patients were required to have continuous eligibility for 6 months prior and 12 months following index. Patients also had ≥1 PsA diagnosis claim in the pre-period or on index and could not have claims for other indicated conditions, except psoriasis. Biologic treatment patterns, including persistence and switching, were examined over the 12-month follow-up within the IL-17Ai and TNFi cohorts.
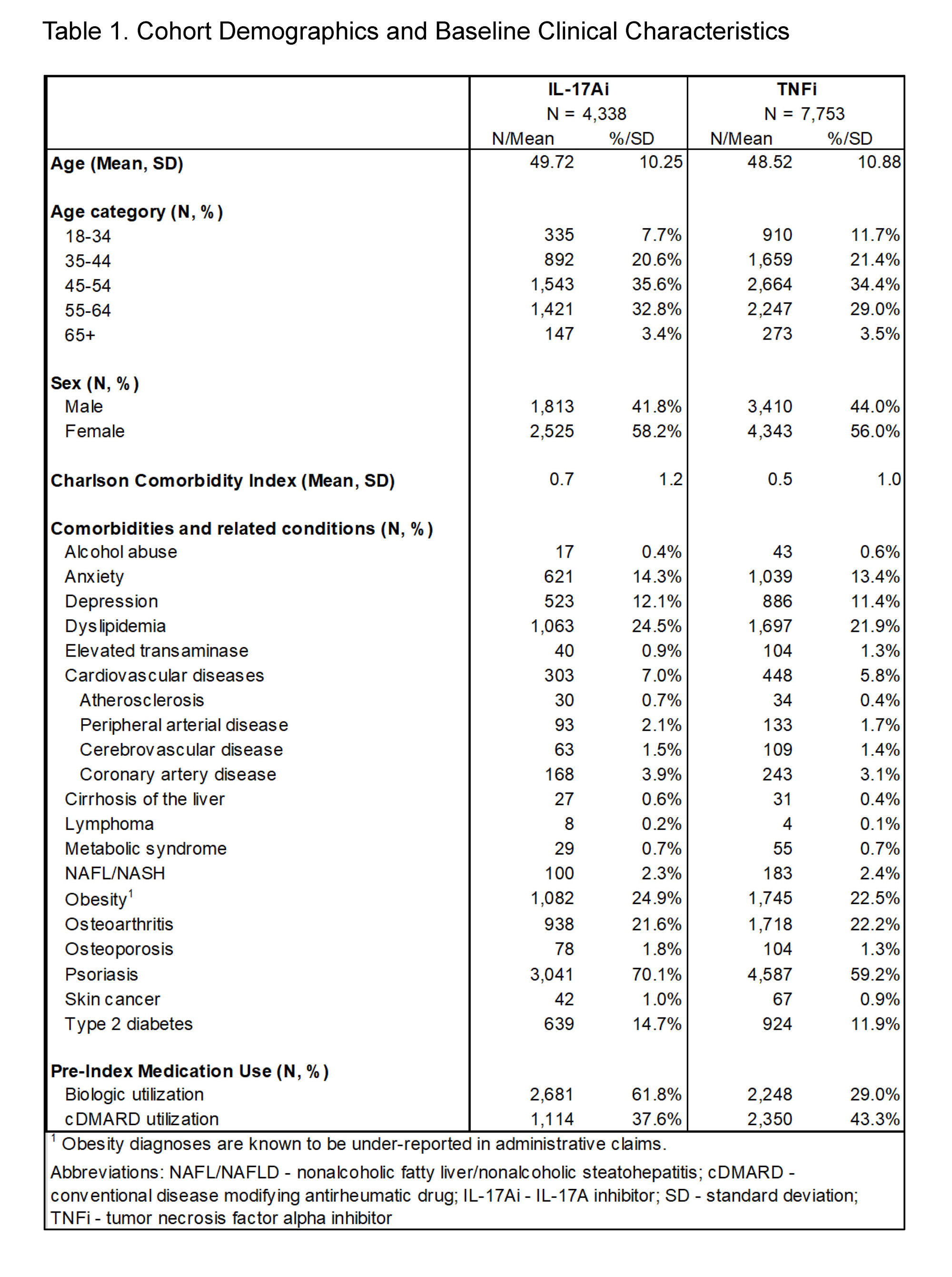
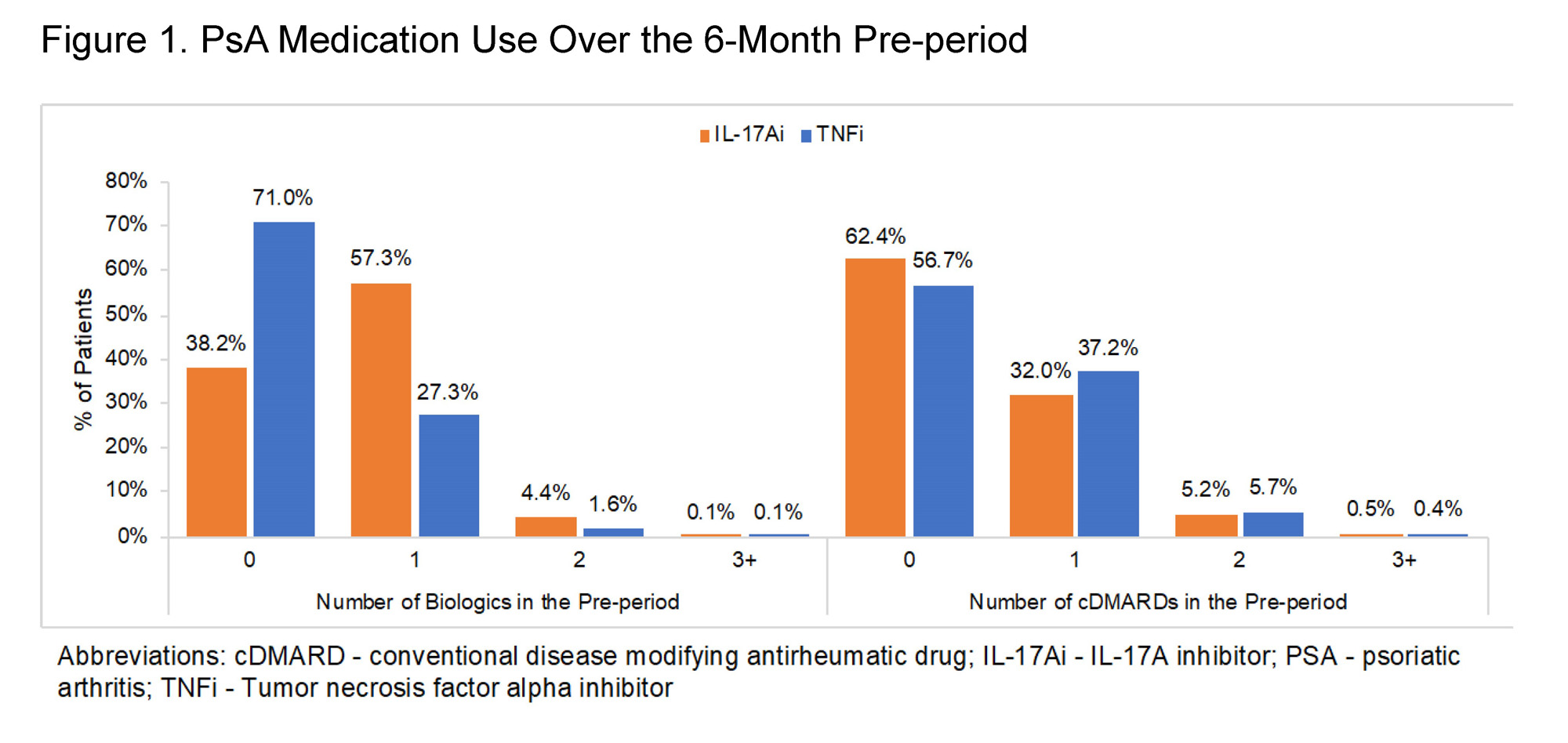
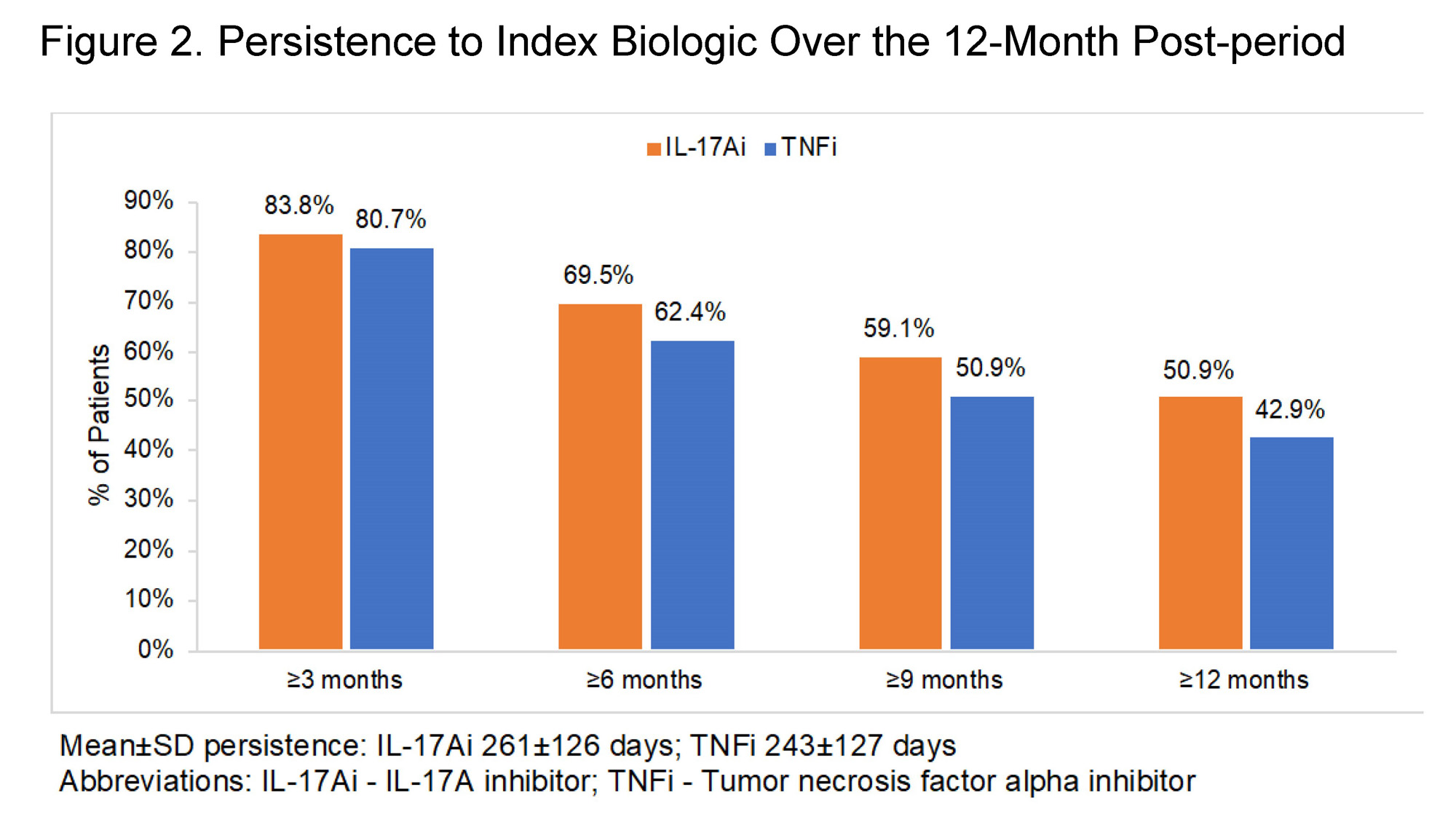
Results: A total of 4,338 IL-17Ai and 7,753 TNFi treated patients were eligible for the analyses. The majority of patients in both cohorts were female and aged 45-64 at index; mean±SD Charlson Comorbidity Index was 0.5±1.0 in the TNFi cohort and 0.7±1.2 in the IL-17Ai cohort (Table 1). Prior biologic use was observed in the baseline period for 29.0% of TNFi patients and 61.8% of IL-17Ai patients, while baseline conventional disease-modifying antirheumatic drug (cDMARD) use was observed for 43.3% and 37.6% of the respective cohorts (Figure 1; Table 1). TNFi or IL-17Ai treatment was initiated as combination therapy with cDMARDs in 22.6% of the TNFi cohort and 17.3% of the IL-17Ai cohort. Over the post-period, IL-17Ai patients remained on an IL-17Ai for a mean persistence of 261±126 days; TNFi patients remained on drug for 243±127 days (Figure 2). Rates of biologic switching over the post-period were 25.3% in the IL-17Ai cohort and 29.3% in the TNFi cohort.
Conclusion: Persistence to therapy can be especially important in chronic, progressive diseases like PsA where patients will likely have to change treatment regimens over the course of disease. Results from this study indicate that IL-17Ai biologics can represent a viable, long-lived treatment choice even among patients with prior biologic experience and high comorbidity burden.
To cite this abstract in AMA style:
Vadhariya A, Ross S, Brady B, Varker H, Thu Tran A, Walsh J. Persistence to Therapy Among Patients with Psoriatic Arthritis Treated with IL-17A or TNFα Inhibitors (IL-17Ai or TNFi) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/persistence-to-therapy-among-patients-with-psoriatic-arthritis-treated-with-il-17a-or-tnf%ce%b1-inhibitors-il-17ai-or-tnfi/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-to-therapy-among-patients-with-psoriatic-arthritis-treated-with-il-17a-or-tnf%ce%b1-inhibitors-il-17ai-or-tnfi/