Session Information
Session Type: Abstract Submissions (ACR)
Persistence on Biologics is Associated with Concomitant Methotrexate Use among Rheumatoid Arthritis Patient
Background/Purpose: Concomitant methotrexate (MTX) is associated with improved treatment efficacy in randomized controlled trials of biologic agents to treat rheumatoid arthritis (RA), yet many patients receive biologics without MTX. The present study compared persistence among RA patients who initiated a biologic alone vs. with MTX in routine clinical practice. We also examined if the association differed by type of biologic initiated.
Methods: We conducted a retrospective cohort study among RA patients using national Medicare administrative claims data from 2006 to 2010. Eligible patients were new users (no use of specific agent in 12 months prior) of etanercept, infliximab, adalimumab, or abatacept and were required to have ≥ 12 months continuous Medicare coverage before (baseline) and after treatment initiation. Exposure groups were biologic monotherapy (no MTX or any other non-biologic disease modifying anti-rheumatic drugs [NB-DMARDs]) versus biologic with MTX (with or without other NB-DMARD). The outcome was non-persistence with biologics, which included discontinuation or switching to a different biologic. Patients were censored if they changed from monotherapy to combination therapy or vice versa. We generated Kaplan-Meier curves to compare biologic non-persistence across different exposure groups. We also calculated the hazard ratio (HR) for biologic non-persistence comparing monotherapy to combination therapy overall and for each biologic agent separately adjusting for demographics, receipt of state subsidy, reason for Medicare enrollment, Charlson co-morbidities, and any hospitalization during baseline.
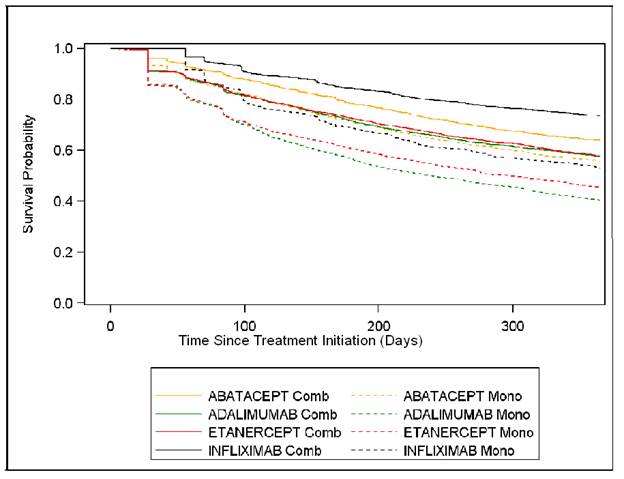
Results: Of 22,073 eligible RA patients, 8,755 initiated biologic monotherapy and 13,318 initiated a biologic in combination with MTX. At treatment initiation, mean age (standard deviation) was 65.6 (12.6) and 82.0% were women. Patients who initiated a biologic without concomitant MTX were 1.7 (95% CI: 1.6-1.8) times more likely to be non-persistent. The magnitude of the association between concomitant MTX use and biologic non-persistence differed significantly by biologic agent (p < .0001); the strongest association was observed among infliximab users (HR: 2.1; 95% CI: 1.9-2.3) and the smallest among abatacept users (HR: 1.5; 95% CI: 1.3-1.6). Kaplan-Meier figure presents detailed data.
Conclusion: Concomitant MTX was associated with better persistence on biologic therapy; the largest impact was observed among infliximab users.
Figure: Kaplan-Meier curves of time to non-persistence by type of biologic therapy and use of concomitant methotrexate
Disclosure:
J. Zhang,
Roche/Genentech,
2;
F. Xie,
None;
E. S. Delzell,
Amgen,
2;
H. Yun,
None;
J. Lewis,
Pfizer, Prometheus, Lilly, Shire, Nestle, Janssen, AstraZeneca, Amgen,
5,
Centocor, Shire, Takeda,
2;
K. Haynes,
None;
L. Chen,
None;
J. R. Curtis,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, crescendo, AbbVie,
2,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, crescendo, AbbVie,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-on-biologics-is-associated-with-concomitant-methotrexate-use-among-rheumatoid-arthritis-patient/