Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Persistence with osteoporosis therapy is important for optimal reduction of fracture risk. Persistence with denosumab was >90% in a randomized clinical trial in women with low bone mineral density (BMD), but persistence in the community setting is undetermined. We are conducting a prospective observational study to evaluate persistence with denosumab in routine clinical practice in the United States (US) and Canada (CAN).
Methods:
In this ongoing multicenter, single-arm study, women were enrolled within 4 weeks after the first injection of subcutaneous denosumab recommended for administration every 6 months for the treatment of osteoporosis per the local product label. Clinical sites include primary care and specialty practices. No clinical procedures, assessments, or changes to routine management of patients are required. Participants are followed for 24 months after study entry. We report results of a 12-month interim analysis. Study endpoints include persistence with denosumab at 12 months (defined as receipt of at least 2 injections no more than 6 months + 8 weeks apart); patient and physician attributes predicting persistence; and occurrence of serious adverse events (SAEs). Persistence rates are based on total patient enrollment.
Results:
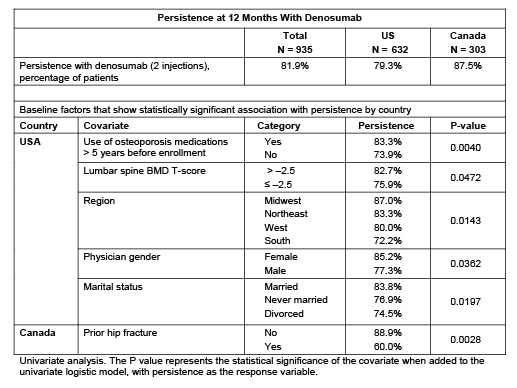
Enrollment was completed in April 2012 (N=935; US n=632; CAN n=303). Of 80 participating physicians (US n=54; CAN n=26), 41% in the US and 19% in CAN are rheumatologists. In the US, 76% of patients were ≥65 years of age at baseline, vs 62% in CAN. The mean (SD) Wolfe comorbidity index was 2.4 (2.0) in the US and 1.9 (1.6) in CAN. More than half of patients (53%) had osteoarthritis, 9% had rheumatoid arthritis, and 9% had other rheumatologic disorders. During the 5 years before enrollment, 92% of patients in both the US and CAN had taken a prescription osteoporosis therapy, and 59% (US 57%; CAN 62%) had used osteoporosis therapy >5 years before enrollment. Patients in the US took a mean (SD) of 8.4 (5.0) prescription medications, vs 5.7 (4.3) in CAN.
At 12 months, 82% of patients were persistent with denosumab (US, 79%; CAN 88%; Table). Several baseline factors in the US and one in CAN showed a statistically significant association with persistence (Table). Of the 118 patients (13%) who had discontinued the study at 12 months, 55 (6%) withdrew consent, 9 (1%) were lost to follow-up, 8 (1%) died, and 46 (5%) cited other reasons. SAEs were reported in 66 patients (7%); no SAEs of osteonecrosis of the jaw (ONJ), atypical femoral fracture, fracture healing complications, hypocalcemia, eczema, or hypersensitivity were reported. AEs of fracture were reported by 21 patients (2%).
Conclusion:
In this study of routine clinical practice, more than 80% of patients for whom denosumab was prescribed persisted on therapy at 12 months. Denosumab appears to be well tolerated; no new safety risks have been identified to date.
Disclosure:
S. L. Silverman,
Amgen Inc, Eli Lilly and Co. Medtronic, Pfizer,
2,
Amgen Inc., Genentech, Eli Lilly and Co., Novartis, Pfizer,
5,
Amgen Inc., Eli Lilly and Co., Pfizer,
8,
Cedars-Sinai Medical Center, Bone Center of Excellence,
3;
E. Siris,
Amgen, Eli Lilly, Merck, Novartis, Pfizer,
5,
Amgen, Lilly ,
8,
Governance Committee, National Bone Health Alliance,
6;
D. L. Kendler,
Amgen, Eli Lilly, Novartis, Pfizer, GlaxoSmithKline, Johnson Johnson,
2,
Amgen, Eli Lilly, Novartis, Pfizer, GlaxoSmithKline,
5,
Amgen, Eli Lilly, Novartis, Pfizer, GlaxoSmithKline,
8;
D. Belazi,
Amgen Inc., Eli Lilly and Co.,
5;
J. P. Brown,
Abbott, Amgen, Bristol-Myers-Squibb, Eli Lilly, Merck, Novartis, Roche, sanofi-aventis, Servier, Takeda, Warner Chilcott ,
2,
Amgen, Eli Lilly, Merck, Novartis, sanofi-aventis, Warner Chilcott ,
5,
Amgen, Eli Lilly, Novartis, Merck ,
8;
D. T. Gold,
Lilly, Amgen,
5,
Lilly, Amgen,
8;
E. M. Lewiecki,
Amgen, Eli Lilly, Merck,
2,
Amgen, Eli Lilly, Merck,
5,
Amgen, Eli Lilly, Merck,
8,
National Osteoporosis Foundation, United States Bone and Joint Initiative, International Society for Clinical Densitometry,
6,
New Mexico Clinical Research & Osteoporosis Center,
3;
A. Papaioannou,
Amgen, Warner Chilcott, Eli Lilly, Merck Frosst,
2,
Amgen, Eli Lilly, Merck Frosst, Warner Chilcott ,
5,
Amgen, Warner Chilcott, Eli Lilly, Merck Frosst,
8;
C. Simonelli,
Amgen Inc., Eli Lilly and Co.,
2,
Amgen Inc., Eli Lilly and Co,
5,
Amgen Inc., Eli Lilly and Co,
8;
I. Ferreira,
Bristol-Myers Squibb, Amgen Inc., Novartis,
1,
Amgen Inc.,
3;
P. Dakin,
Amgen Inc.,
1,
Amgen Inc.,
3;
S. Siddhanti,
Amgen Inc.,
1,
Amgen Inc.,
3;
B. Stolshek,
Amgen Inc.,
1,
Amgen Inc.,
3;
C. Recknor,
Ion Med Systems,
1,
Novartis Pharmaceutical Corporation,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-at-12-months-with-denosumab-prolia-in-postmenopausal-women-with-osteoporosis-interim-results-from-a-prospective-observational-study/