Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Registry data regarding biologic DMARD therapy as a mono or combo (combined with a traditional oral DMARD) in subjects with Psoriatic Arthritis (PsA) are limited. Our aim was to characterize biologic naïve PsA patients who initiated TNFi as mono or combo therapy, estimate length of time on initial TNFi and identify characteristics associated with longevity of initial TNFi use.
Methods: Data from the US Corrona registry was used. Patients (pts) with a diagnosis of PsA ≥ 18 yrs of age, bio-naïve and initiated TNFi on Jan 1st 2005 or later with at least 3m of follow-up were included. Survival analyses were performed using Kaplan-Meier curves estimating time on initial therapy since TNFi initiation, either as mono/combo therapy. Proportional hazard models were used to identify factors associated with risk of therapy change since initiation. A propensity score (PS) for mono vs combo was estimated and comparisons made using inverse probability weighting based on propensity probabilities. Median time to therapy change was calculated.
Results: 519 biologic naïve PsA pts met the inclusion criteria, with 61% vs. 39% initiating a TNFi as combo or mono therapy, respectively. 51% of pts initiating a TNFi were female, mean age was 51.6 yrs and mean disease duration of 6.3 yrs. The combination therapy group had significantly higher proportion of women (57.1% vs 42.3%), higher clinical disease activity index (CDAI) (mean (SD): 12.5 (11.1) vs 9.6 (9.9)), higher body mass index (mean(SD) 31.9 (7.4) vs 30.7 (6.5), higher proportion of history of diabetes (11.3% vs 6.5%) and higher history of methotrexate use (89.9% vs 68.2%) compared to monotherapy initiators.
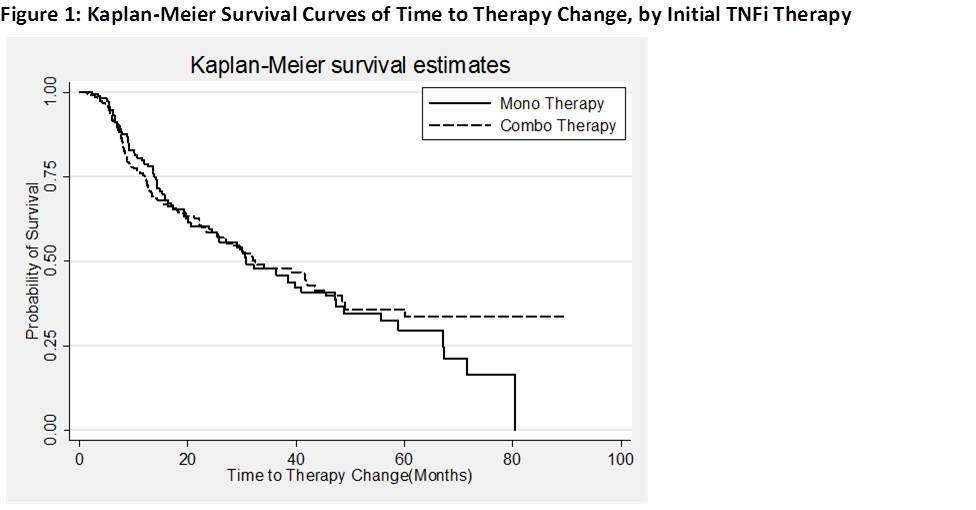
Median time on combo and mono therapy since TNFi initiation in the PS matched pts was 32.8 months and 30.8 months respectively [Figure 1]. Significant factors associated with change in initial therapy were disability index measured by mHAQ (HR (95% CI): 2.6 (1.7-3.9)) and CDAI (HR (95% CI): 1.03 (1.01-1.04)). The models were adjusted for age, gender, body mass index, alcohol use, smoking history, mHAQ, and whether the patient was on mono/combo therapy. Persistency for mono vs combo therapy differed by individual TNFi. For example, pts on ETN mono therapy had higher persistency than combo therapy (47.3m on mono vs 19.1m in combo) with a HR of 1.93 (95% CI, 1.15-3.25). Among the pts on IFX, combo therapy was more persistent with a HR of 0.46 (95% CI, 0.24-0.88).
Conclusion: Over a third of PsA pts in the Corrona registry initiated TNFi as monotherapy. Even when adjusting for channeling bias with PS, overall survival on TNFi therapy was similar between mono and combo-treated pts. Greater degree of disability and disease activity use were associated with shorter duration of mono therapy. Some differences in survival on drug were noted between TNFi medicines, potentially related to background disease severity, immunogenicity, and/or other factors.
Disclosure:
P. J. Mease,
Celgene, Merck, Novartis, Abbvie, Amgen, BiogenIdec, BMS, Genentech, Janssen, Lilly, Pfizer, UCB;,
2,
Celgene, Merck, Novartis, Abbvie, Amgen, BiogenIdec, BMS, Crescendo, Genentech, Janssen, Lilly, Merck, Pfizer, UCB, Vertex;,
5,
Abbvie, Amgen, BiogenIdec, BMS, Crescendo, Genentech, Janssen, Lilly, Pfizer, UCB,
8;
D. Collier,
Amgen,
3,
Amgen,
1;
C. Karki,
Corrona, LLC.,
3;
G. Li,
Axio Research, LLC.,
3;
B. Bitman,
Amgen,
1,
Amgen,
3;
J. D. Greenberg,
Corrona, LLC.,
1,
Corrona, LLC.,
3,
AstraZeneca, Celgene, Novartis and Pfizer,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-and-predictors-of-biologic-tnfi-therapy-among-biologic-naive-psoriatic-arthritis-patients-in-a-us-registry/