Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic disease that causes inflammation of the joints, entheses, spine, skin, and nails.1 While available advanced treatments (txs) for PsA have expanded, many patients (pts) have suboptimal responses despite undergoing multiple lines of biologic or targeted synthetic DMARDs (b/tsDMARDs).2 This study evaluated tx persistence (time to discontinuation) and change from baseline (CfB) in disease activity and pt-reported outcomes (PROs) among pts with PsA following initiation of a third-line or higher (3L+) b/tsDMARD.
Methods: This retrospective cohort study included pts who were ≥18 years old, had a PsA diagnosis, and initiated a 3L+ b/tsDMARD between January 2016–December 2023, at or after entry into the CorEvitas PsA/ Spondyloarthritis Registry (baseline), a prospective, multicenter, observational US registry. Persistence was defined as time from therapy initiation to discontinuation (with or without switch); pts were censored at the last available registry visit within 24 months after initiation or end of the study period, whichever occurred first. CfB in PsA disease activity and PRO measures were assessed after the 6-month follow-up period. Continuous variables were reported as mean (standard deviation [SD]) and median (interquartile range [IQR] or 95% confidence intervals [CIs]). Categorical variables were described with counts and percentages.
Results: The registry included 7,044 b/tsDMARD initiations, with 2,550 (36.2%) being 3L+. This study included 1,210 pts initiating their first 3L+ b/tsDMARD; of these, 637 had a 6‑month follow-up. Of the 1,210 pts included in this analysis, mean (SD) age was 54.2 (12.3) years and median (IQR) PsA duration was 7.0 (3.0, 12.0) years (Table 1). At b/tsDMARD initiation, mean (SD) swollen joint count (SJC) and tender joint count (TJC) were 2.7 (4.4) and 7.7 (10.3), respectively. Baseline PROs demonstrated moderate‑to-high disease severity. Mean (SD) Health Assessment Questionnaire Disability Index (HAQ-DI) score was 0.9 (0.7). Mean (SD) pain and fatigue Visual Analog Scale (VAS)-100 scores were 54.0 (27.7) and 53.8 (28.0), respectively (Table 1). Median (95% CI) tx persistence was 13.4 (12.4, 15.2) months (Figure 1); 76.0% (95% CI: 73.0, 78.0), 54.0% (95% CI: 51.0, 57.0), and 42.0% (95% CI: 39.0, 45.0) of pts remained on therapy at 6, 12, and 18 months, respectively. Among pts with 6-month follow-up (n=637), statistically significant improvements from baseline occurred in most disease activity and PRO measures except for dactylitis count, HAQ-DI, EuroQoL 5-dimensional, 3-level (EQ-5D-3L), and Work Productivity and Activity Impairment (WPAI) absenteeism (Table 2).
Conclusion: While median tx persistence greater than 1 year was observed, suboptimal improvements were reflected across disease activity measures and quality of life-related PROs, suggesting that pts may experience insufficient disease control even after multiple lines of tx. Therefore, earlier, more effective tx options are needed to mitigate the development of persistent symptoms and achieve disease control.
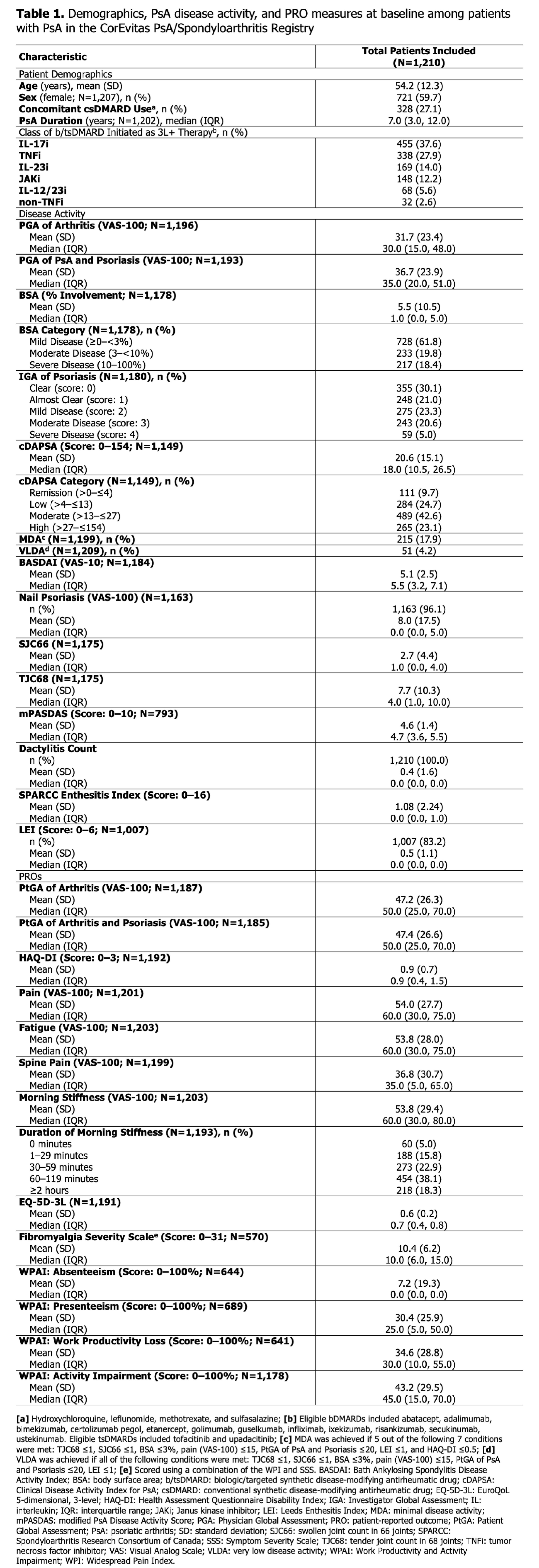 Table 1. Demographics, PsA disease activity, and PRO measures at baseline among patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry
Table 1. Demographics, PsA disease activity, and PRO measures at baseline among patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry
.jpg) Figure 1. Kaplan-Meier curve of treatment persistence among patients with PsA initiating a 3L+ b/tsDMARD therapy
Figure 1. Kaplan-Meier curve of treatment persistence among patients with PsA initiating a 3L+ b/tsDMARD therapy
.jpg) Table 2. CfB in disease activity and PRO measures following initiation of a 3L+ b/tsDMARD to 6-month follow-up among patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry
Table 2. CfB in disease activity and PRO measures following initiation of a 3L+ b/tsDMARD to 6-month follow-up among patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry
To cite this abstract in AMA style:
Mease P, Middaugh N, Muñoz Maldonado Y, Song C, Eliot M, Low R, Ogdie A. Persistence and Disease Activity Control among Patients with Psoriatic Arthritis in the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry Initiating a Third or Higher Line of Biologic or Targeted Synthetic Disease-Modifying Antirheumatic Drug Therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/persistence-and-disease-activity-control-among-patients-with-psoriatic-arthritis-in-the-corevitas-psoriatic-arthritis-spondyloarthritis-registry-initiating-a-third-or-higher-line-of-biologic-or-target/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-and-disease-activity-control-among-patients-with-psoriatic-arthritis-in-the-corevitas-psoriatic-arthritis-spondyloarthritis-registry-initiating-a-third-or-higher-line-of-biologic-or-target/
