Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: B cell subset numbers, especially lower memory B (Bmem) cells, predict clinical responsiveness to rituximab in several diseases including pemphigus and rheumatoid arthritis (RA). The Rituximab in Myositis (RIM) trial enrolled 200 refractory myositis subjects (76 dermatomyositis (DM), 76 polymyositis (PM), 48 juvenile dermatomyositis (JDM)). Our aim was to determine whether the numbers and relative percentage of peripheral blood B cell subsets at baseline and after rituximab predicted clinical responses in refractory myositis.
Methods: Using flow cytometry, we analyzed total B cell numbers and B cell subset (transitional/naïve/switched and unswitched Bmem/ and plasmablast) percentages at baseline, 8, 24 and 32 or 36 weeks after rituximab. We assessed 71 “responders” who met the study definition of improvement (DOI) without experiencing subsequent disease worsening and compared them to 35 “non-responders.” The DOI was defined as 20% improvement in at least 3 of 6 core set disease activity measures, including MD and subject global disease activity, manual muscle testing (MMT), physical function, muscle enzymes, and extramuscular disease activity. We used Mann-Whitney tests to determine the association of B cell subsets with response. Cox proportional hazard models were used to determine if B cell subsets predicted a better (i.e. shorter) time to response post rituximab. Spearman correlations assessed the relationship of changes in MMT and MD global post rituximab to changes in Bmem and plasmablast subsets.
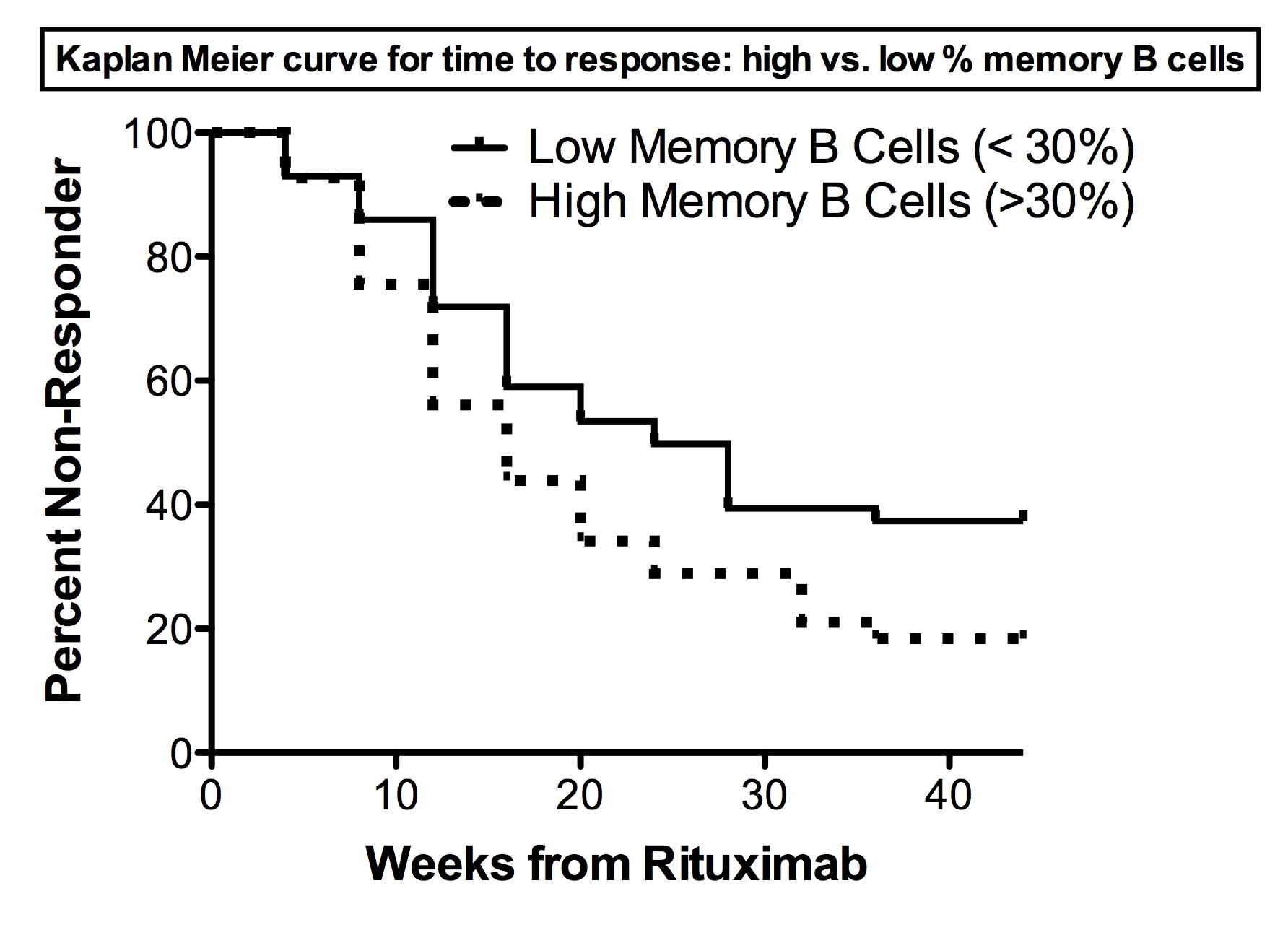
Results: 97% of RIM subjects depleted their B cells to <5/ul following rituximab. There was no difference in total B cell numbers, total and % B cell subsets, or the ratios of transitional and naive:Bmem cells between responders and non responders at any time-point. There was no correlation between change in B cell subsets and the change in MD global disease activity or MMT. However, among myositis autoAb positive subjects (N=80; 58 responders, 22 non-responders), there was a significantly higher percentage of Bmem cells in responders at week 8 [median (IQR): 30.6% (17.8-45.9) vs. 21.3% (12.8–26); p = 0.009], mainly due to a higher percentage of switched Bmem cells. The total number of Bmem cells at week 8 showed a similar trend (p=0.02). The percentage of Bmem cells at week 8 was also associated with a shorter time to response (p=0.008; Figure 1), mainly due to switched memory B cells in autoAb positive subjects (p=0.004). Similar trends between clinical responsiveness and Bmem cell numbers were seen in the overall cohort as in the PM, DM, and JDM subsets but not statistically significant.
Conclusion: In contrast to RA, a higher percentage of Bmem cells at week 8 was associated with clinical responsiveness in autoAb positive myositis patients post rituximab. This suggests the presence of a subpopulation of Bmem cells in myositis associated with a favorable response following B cell depletion.
Disclosure:
R. Aggarwal,
None;
C. V. Oddis,
Genentech and Biogen IDEC Inc.,
2,
Genentech and Biogen IDEC Inc.,
5;
E. R. Wilkerson,
None;
D. Koontz,
None;
I. D. Metes,
None;
A. M. Reed,
None;
D. P. Ascherman,
None;
M. C. Levesque,
Genentech and Biogen IDEC Inc.,
2,
Genentech and Biogen IDEC Inc.,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/peripheral-blood-memory-b-cell-numbers-predict-clinical-response-following-rituximab-treatment-of-adult-and-childhood-myositis/