Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: SB2 is approved in the EU as an infliximab (IFX) biosimilar, having demonstrated bioequivalence and similar efficacy, safety and immunogenicity as the reference. There is limited real-world evidence published on persistence or safety of SB2, either in IFX-naiive patients or in those transitioning from originator or another IFX biosimilar. PERFUSE is an ongoing non-interventional study intending to enroll over 1,000 patients receiving SB2 as routine therapy, with the objective of describing clinical characteristics, effectiveness, treatment persistence and safety in patients initiating SB2 in routine clinical practice and followed for 12 months at 6 specialist rheumatology sites across France.
Methods: Eligible adult patients have a diagnosis of Rheumatoid Arthritis (RA), Psoriatic Arthritis (PsA) or Ankylosing Spondylitis (AS) and initiated SB2 in routine clinical practice after September 2017, either as their first IFX or transitioning from treatment with IFX originator or another IFX biosimilar. Data are captured prospectively and/or retrospectively from patient records obtained during routine clinic visits for up to 12 months (M12) following initiation. Outcome measures include persistence on SB2, clinical characteristics at baseline (time of initiation of SB2), disease scores (DAS28, BASDAI and Serious Adverse Events (SAEs).
Results: This interim analysis (IA) includes 177 patients (41 with RA, 23 with PsA and 113 with AS); of these, 24 patients with RA, 8 patients with PsA and 62 patients with AS reached M12 by the data extraction date. Persistence on SB2 at M12 was 100% (95% CI 85.8, 100), 100% (95% CI 63.1, 100) and 87% (95% CI 76, 94.2) in RA, PsA and AS respectively. In the patients with prior IFX, no clinically meaningful difference in disease activity score from baseline to M6 was observed; BASDAI mean individual change was -1.9 (95% CI -3.6, -0.1) in AS (n= 59) and DAS28 mean individual change was 0.1 (95% CI -0.4, 0.7) in RA (n=30). Low sample size precluded effectiveness analysis of IFX-naiive patient data. Four SAEs were reported: One related to SB2 (infected cyst) and three unrelated (two RA disease flares and one overdose of vitamin K antagonists).
Conclusion: This IA indicates that patients with chronic inflammatory rheumatism can be successfully transitioned from originator or biosimilar IFX to SB2, with no loss of disease control and without safety concerns. Over 85% of patients initiated de novo or transitioned from originator or another IFX biosimilar continued SB2 treatment at M12 post-initiation. Subsequent to these preliminary data, the study will provide ongoing, pertinent information about long-term outcomes in these populations, helping to inform evidence-based treatment decisions.
Acknowledgements:
Biogen International GmbH funded and sponsored this study. Authors had full editorial control and provided final approval of all content.
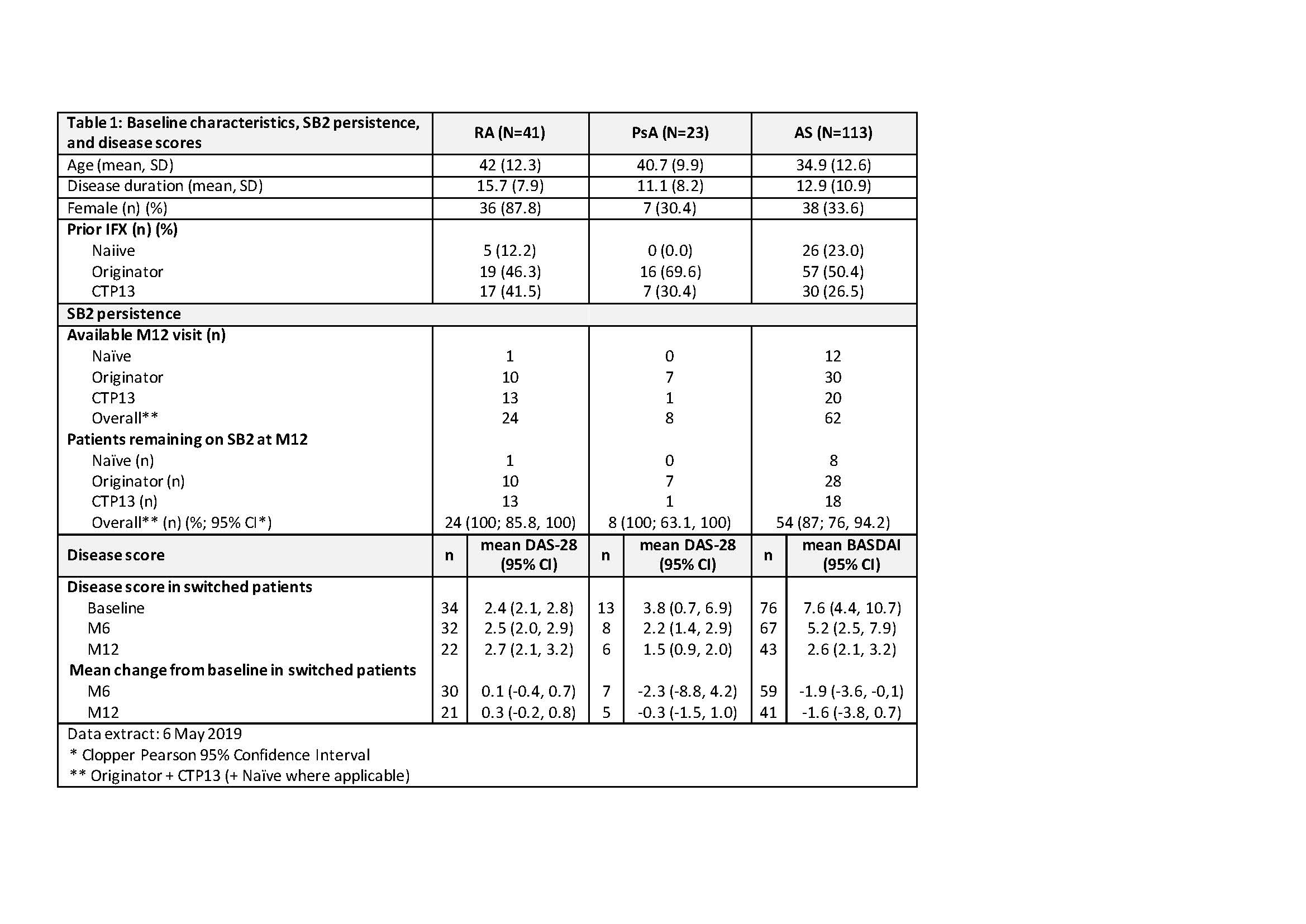
Table 1 -to go in Results section-
To cite this abstract in AMA style:
Fautrel B, Bouhnik Y, Desjeux G, Freudensprung U, Addison J, Brigui A. PERFUSE: A French Prospective/Retrospective Non-interventional Cohort Study of Infliximab-naïve and Transitioned Patients Receiving Infliximab Biosimilar SB2; An Interim Analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/perfuse-a-french-prospective-retrospective-non-interventional-cohort-study-of-infliximab-naive-and-transitioned-patients-receiving-infliximab-biosimilar-sb2-an-interim-analysis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/perfuse-a-french-prospective-retrospective-non-interventional-cohort-study-of-infliximab-naive-and-transitioned-patients-receiving-infliximab-biosimilar-sb2-an-interim-analysis/
