Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The Patient-Reported Outcomes Measurement Information System (PROMIS) was developed to improve measurement of patient-reported outcomes. A large study from the US reported guarded support for the use of PROMIS-29 in RA, OA, FM and SLE patients1. Validity of PROMIS-29 for assessing quality of life in an Australian limited scleroderma cohort was demonstrated by comparison to SF36 and the HAQ-DI2. Our objective was to examine performance of the static 29 item PROMIS profile (PROMIS-29) compared to two legacy disease activity measures in RA, the CDAI and the RAPID3. We report a cross-sectional analysis of PROMIS-29 domain T scores according to CDAI and RAPID3 high, moderate, low and remission disease activity scores in a cohort of Australian patients with RA.
Methods: All patients attending one academic Rheumatology center complete the Multidimensional Health Assessment Questionnaire (MDHAQ) and PROMIS29 questionnaires at each visit. PROMIS29 questionnaires are scored on a 0 to 100 scale normed to the US population and reported as a T score (mean=50; 1 standard deviation (SD) =10). RAPID3 scores (range=0-30) are the sum of the patient physical function score (0-10), patient global assessment (0-10 VNS) and physician global assessment (0-10 VNS) derived from the MDHAQ. CDAI (range=0-76) is the sum of the patient global assessment (0-10 VAS), physician global assessment (0-10 VAS), tender joint count (0-28) and swollen joint count (0-28). T scores were compared across CDAI and RAPID3 disease activity (DA) categories by ANOVA. Minimum clinically important differences (MCIDs) from the population mean of 50 were estimated at 0.5 of the population SD, equivalent to 5 points3.
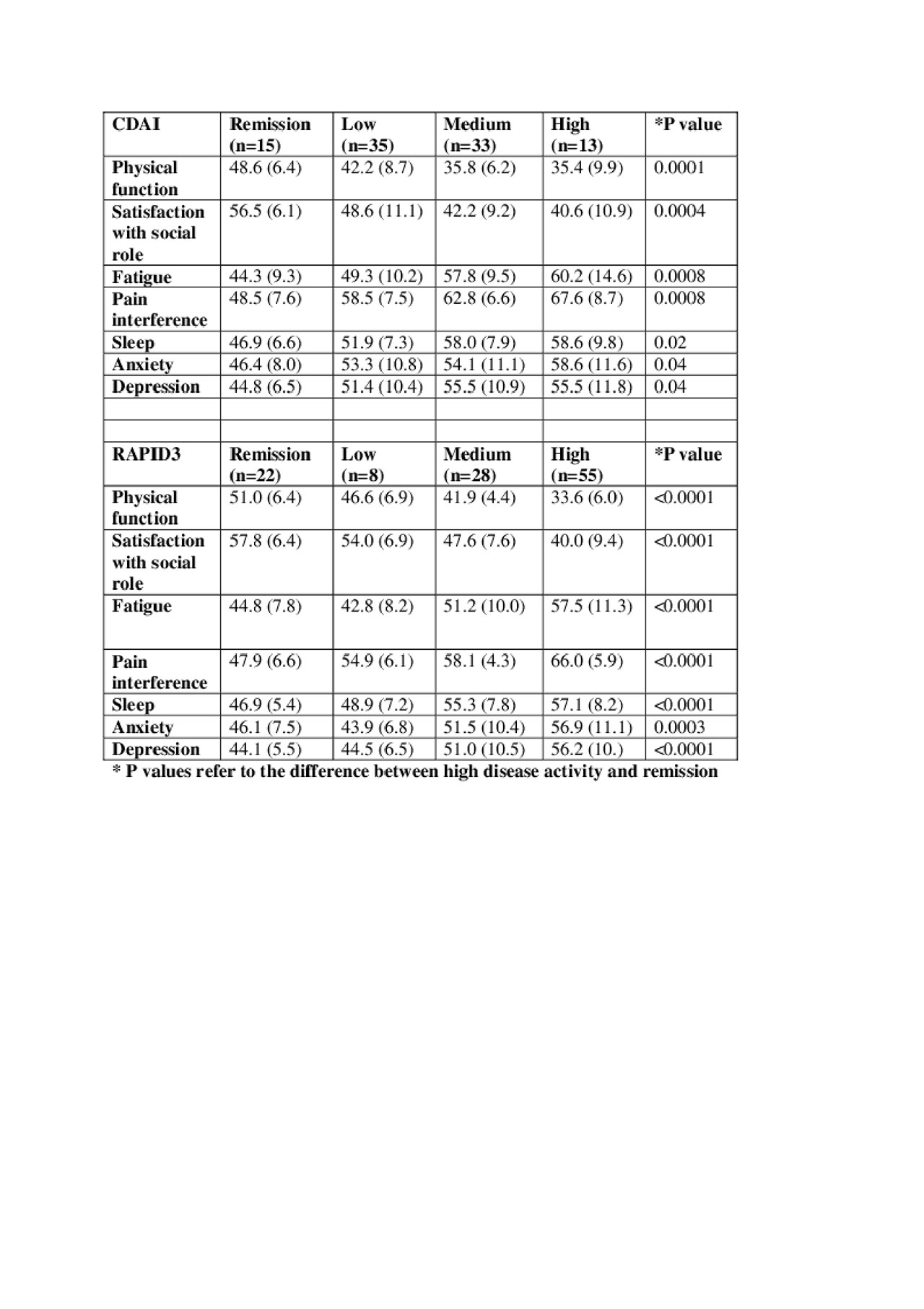
Results: The mean (SD) CDAI was 12.4+/-10.4 with 15.6% in remission, 36.5% in low DA, 34.4% in moderate DA and 13.5% in high DA. All PROMIS domains were significantly worse in patients with high CDAI DA ( >22) compared to remission (≤2.8) (Table). The mean (SD) RAPID3 score was 12.0+/-7.9 with 19.5 % in remission, 7.1% in low DA, 24.8% in moderate DA and 48.7% in high DA. All PROMIS domains were significantly worse in patients with high RAPID3 DA ( >12) compared to remission (≤3.0) (Table). The majority of PROMIS scores in the high and moderate disease activity groups (23 out of 28) by CDAI and RAPID3 met MCID for worse scores than the population mean. Patients in CDAI or RAPID3 remission had PROMIS T scores equivalent to or better than the population mean.
Conclusion: All PROMIS domains in people with high RAPID3 or CDAI disease activity were significantly worse than in people in RAPID3 or CDAI remission. As expected, most PROMIS scores in those with high and moderate disease activity met MCID for being worse than the population mean. Further examination with larger patient numbers in each CDAI and RAPID3 DA category is required to confirm these findings.
- Katz P et al. (2017) Arthritis Care & Research 69(9):1312-1321
- Morrisroe K et al. (2017) J Scleroderma Related Disord 2(3):188-195
- Norman G et al. (2003) Med Care 41:582-592
PROMIS29 vs CDAI and RAPID3 table.docx
To cite this abstract in AMA style:
Gibson K, Gerogevsky D, Huang F, Fang R, Descallar J. Performance of the Patient Reported Outcomes Measurement Information System 29-item Profile in Comparison to the Clinical Disease Activity Index (CDAI) and Routine Assessment of Patient Index Data 3 (RAPID3) in an Australian Rheumatoid Arthritis Cohort [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/performance-of-the-patient-reported-outcomes-measurement-information-system-29-item-profile-in-comparison-to-the-clinical-disease-activity-index-cdai-and-routine-assessment-of-patient-index-data-3/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-the-patient-reported-outcomes-measurement-information-system-29-item-profile-in-comparison-to-the-clinical-disease-activity-index-cdai-and-routine-assessment-of-patient-index-data-3/
