Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Lupus anticoagulant (LA) test performance is critical for APS diagnosis and management. However, variability remains a challenge in LA testing, particularly in anticoagulated samples, with the activated partial thromboplastin time and dilute Russell’s viper venom time (DRVVT) the most commonly used tests. The prothrombin-activating Taipan snake venom time (TSVT) screening test and Ecarin clotting time (ECT) confirmatory test are insensitive to vitamin K antagonists (VKA) and may offer improved LA detection. APS ACTION is an international research network involving 34 centres, a prospective registry and repository, and five core laboratories (CL) worldwide. The aim of this study was to evaluate the performance of the TSVT/ECT in VKA-anticoagulated samples between APS ACTION CLs and examine agreement in LA status with the DRVVT.
Methods: Four CL (A-D) used the same analyser, protocol, and lot of reagents. The manufacturer’s cut‐off values for TSVT/ECT were verified in each CL using plasmas from at least 40 healthy normal subjects, to determine normal ranges for TVST, ECT and TVST/ECT ratios (Diagnostic Reagents Ltd). Results were normalised with pooled normal plasma (PNP), while equal volume mixtures of patient and PNP were tested to confirm the presence of an inhibitor. George King LA Positive plasma and IL LA Negative control plasma (Werfen) were used for quality control (QC). In a validation exercise, each CL tested six positive and six negative blinded VKA-anticoagulated samples, previously identified as such, with status confirmed by a second CL. VKA-anticoagulated samples were also tested with DRVVT Screen and Confirm (Werfen) in 50:50 mixtures with PNP. A precision study was also performed, testing LA-positive and LA-negative QC plasma x 6 in the same run, to determine intra-assay variability.
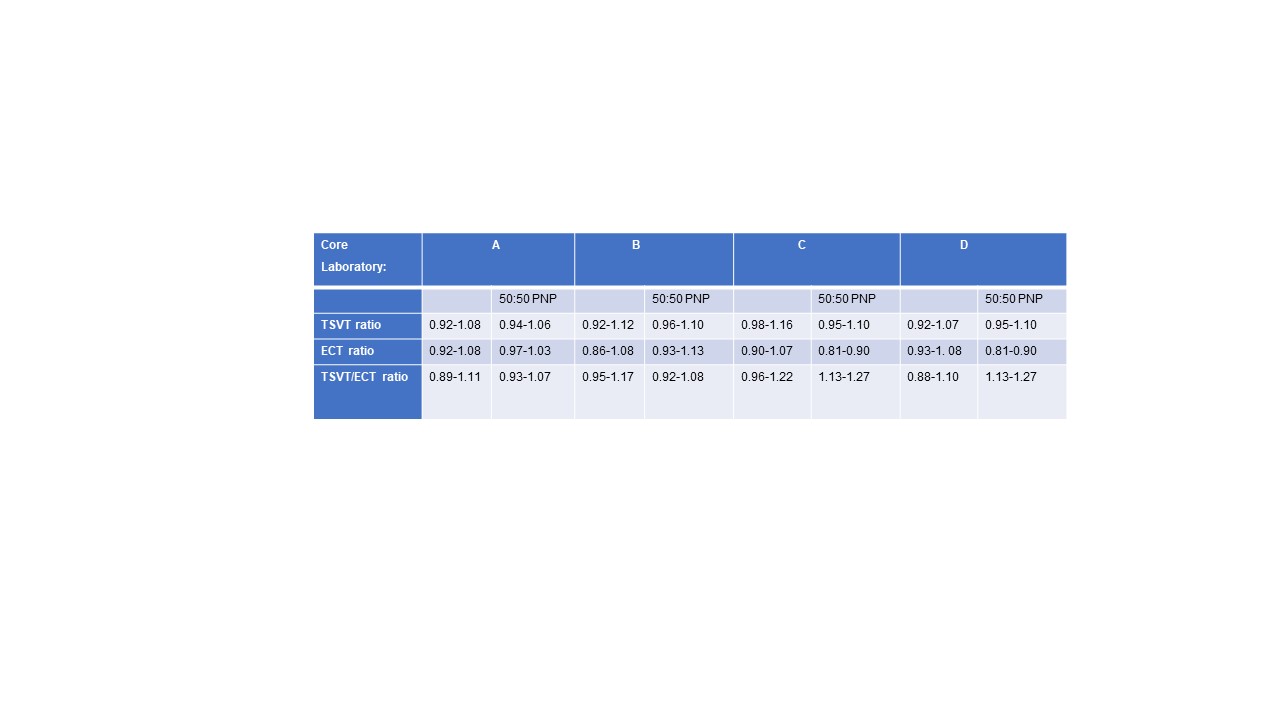
Results: Precision and agreement were acceptable in all CL for both positive and negative control plasma [Coefficient of Variation (CV) 0.7-5%]: CV for clotting times ≤2.5% for LA Negative control plasma and ≤4% for George King LA Positive plasma, CV for TSVT/ECT < 4% for all CLs All CL confirmed the manufacturer’s reference range with minor differences (Table 1). All CL correctly identified the six positive and six negative blinded LA samples (CL D identified one LA positive sample as equivocal). In the ongoing patient sample testing phase of this study, 134 VKA anticoagulated samples showed an overall 66.4% agreement between DRVVT and TSVT/ECT LA status. When results were subcategorised according to INR, agreement was 70.6% for INR <2.0, 66.6% for INR 2.0-3.0, 75.7% for INR 3.0-4.0, and 42.9% for INR >4.0. Of the 45 overall samples with a disagreement between DRVVT and TSVT/ECT, 80% were identified as positive with TSVT/ECT.
Conclusion: LA testing in VKA-anticoagulated samples using the TVST/ECT has been established in four international APS ACTION CL. LA status determined to be negative with DRVVT but positive with TSVT/ECT might be due to dilution of the inhibitor in the 50:50 mixture in the DRVVT, required for clotting factor level normalisation. Preliminary results suggest that the TVST/ECT could provide an adjunctive test to DRVVT testing without the need for mixing studies and with high specificity.
To cite this abstract in AMA style:
Efthymiou M, Mackie I, Willis R, Pengo V, Andrade D, Bison E, Amengual O, Romay-Penabad Z, Fujieda Y, Bertolaccini M, Erkan D, Cohen H, Of APS ACTION O. Performance Assessment of Lupus Anticoagulant Tests Using Taipan and Ecarin Snake Venom Clotting Times: An International Study from Antiphospholipid Syndrome Alliance for Clinical Trials and International Networking (APS ACTION) Core Laboratories [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/performance-assessment-of-lupus-anticoagulant-tests-using-taipan-and-ecarin-snake-venom-clotting-times-an-international-study-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-internati/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-assessment-of-lupus-anticoagulant-tests-using-taipan-and-ecarin-snake-venom-clotting-times-an-international-study-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-internati/