Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: There is currently a lack of consensus among experts on the optimal therapeutic management of psoriatic arthritis (PsA). EULAR and GRAPPA recommendations support initial treatment with classic synthetic (cs)DMARDs. Recently the American College of Rheumatology /National Psoriasis foundation have presented draft recommendations suggesting that treatment naïve patients with active PsA be treated with anti-TNF agents first. In clinical practice it is assumed that treatment with a csDMARD, usually methotrexate (MTX), precedes therapy with biologic agents. In this analysis we evaluated whether the current pattern of PsA treatment in a validated academic cohort of PsA patients begins with MTX.
Methods: All patients with ICD-9 or -10 codes for PsA seen at a single academic center between January 1, 2013 and December 31, 2016 were identified. Patients who met CASPAR classification criteria and consented were enrolled. Patients were sent a survey eliciting date of PsA diagnosis, medication use, efficacy of therapy and reason for stopping medications.
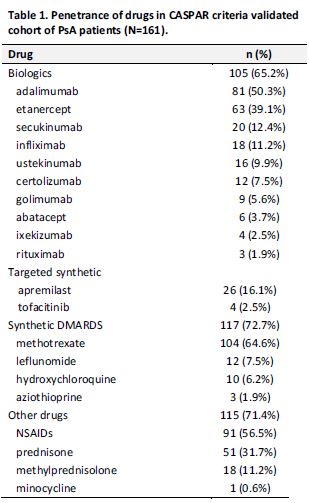
Results: 161/336 (47.9%) of patients completed the survey: mean age 58.3 years (range 22-100), 50.3% female, 88.2% white, 3.4% Hispanic. 64.6% had been on MTX at some point in time and of these, 32.4% were currently using MTX. 35.4% had never been on MTX. 88.2% of patients who took MTX received it without ever being prescribed a biologic or prior to starting a biologic; of these 36.6% were never prescribed a biologic. Of the patients still on MTX, 84.4% took MTX orally and 15.6% via injection, with a mean oral MTX dose of 15.3mg weekly (range 2.5-25). 83.9% of patients who were currently taking MTX believed it helped their PsA, and 43.1% of patients who stopped MTX believed it had helped their PsA. Overall 77.6% of patients who were once on MTX discontinued; 46.4% because MTX was ineffective and 40.6% because of side effects, fatigue being the most frequent (See Table 2). 26.1% of patients had at some point been on a non-MTX csDMARD. 65.2% of patients had been on a biologic agent at some point in time, and of these 56.2% have taken ≥2 biologic agents. 48.4% of patients were currently taking a biologic agent.
Conclusion: Nearly 9 out of 10 patients in an academic PsA Registry received MTX prior to a biologic, and 32.4% of MTX users remained on it. Interestingly, among those who discontinued, 43.1% believed it helped their PsA. These patterns suggest a benefit from MTX, and that even in a center where patients and physicians have access to biologics, MTX remains standard of care as initial therapy. Given the lower cost and patient self-report of benefit, in selected patients MTX should remain a first-line treatment.
To cite this abstract in AMA style:
Epsten MJ, Mandl LA, Szymonifka J, Schwartzman S. Patterns of Medication Use in a Validated Cohort of Psoriatic Arthritis (PsA) Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/patterns-of-medication-use-in-a-validated-cohort-of-psoriatic-arthritis-psa-patients/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-medication-use-in-a-validated-cohort-of-psoriatic-arthritis-psa-patients/