Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Sarcoidosis results in significant morbidity and mortality but is a generally understudied disease. There are currently no FDA approved drugs for the treatment of extra-pulmonary sarcoidosis, but rheumatologists use a variety of treatment strategies in this patient population. We used the ACR’s RISE registry to analyze the spectrum of drugs used to treat sarcoidosis in real-world practice.
Methods: RISE is a national, EHR-enabled registry that passively collects data on all patients seen by participating practices, reducing the selection bias present in single-insurer claims databases. As of December 2017, RISE held validated data from 1,257 providers in 236 practices, representing ~36% of the U.S. clinical rheumatology workforce. Patients included in this study were ≥18 years old, had medication data available, and had ≥ 1 code for sarcoidosis between January 1 2017 and December 31 2017. We identified sarcoid-related disease manifestations during the calendar year including eye, lung, and joint involvement according to validated ICD code definitions. We assessed medication use, including conventional synthetic DMARDs, biologic DMARDs, new synthetic DMARDs, and glucocorticoids during 2017; patients were considered treated if they received ≥ 1 prescription for a drug from a given category.
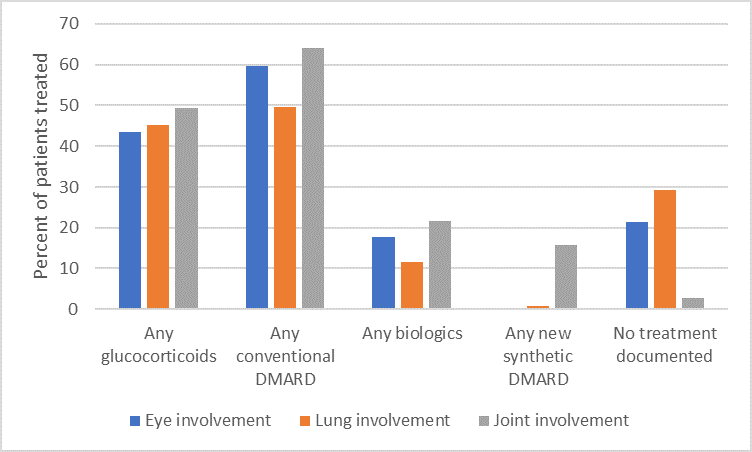
Results: We found 3791 unique patients with ≥ 1 diagnosis of sarcoidosis from 188 practices. 77% were female; 50% white, 29% black; with mean age 58.2 ± 12.4. Specific manifestations included eye disease (3.6%), lung disease (24.7%), and joint disease (29.9%). Overall, 2094 (55.2%) were treated with at least one DMARD of any type. 13.5% received a biologic or new synthetic DMARD, the most common of which were TNF inhibitors (76.4% of this category; top drugs infliximab and adalimumab), followed by rituximab (5.2%), tofacitinib (5.0%), and abatacept (4.6%). Biologics and new synthetic DMARDs were most often prescribed for patients with joint involvement (see Figure). However, overall, 579 patients (15.3%) were prescribed glucocorticoid monotherapy; 597 (15.7%) were prescribed prolonged glucocorticoid therapy (prednisone 10 mg or equivalent for longer than 90 days). No significant variation was identified according to race, insurance status or geographic region after controlling for specific disease manifestations.
Conclusion: To our knowledge this is the first study to assess patterns of medication use for patients with sarcoidosis seen in U.S. rheumatology practices. Given that there are no approved drugs for extra-pulmonary sarcoidosis, the meaningful use of TNFi suggests that they have become standard of care, especially for the joint manifestations of this disease. Our finding that 15% of patients have prolonged use of moderate or high dose glucocorticoids suggests that there may be a significant need for new drug research and development in this area.
Disclaimer: This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
To cite this abstract in AMA style:
Schmajuk G, Kay J, Li J, Choden S, Morgan E, Yazdany J, Reimold A. Patterns of Medication Use for Patients with Sarcoidosis: Data from the ACR’s RISE Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/patterns-of-medication-use-for-patients-with-sarcoidosis-data-from-the-acrs-rise-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-medication-use-for-patients-with-sarcoidosis-data-from-the-acrs-rise-registry/