Session Information
Date: Monday, November 13, 2023
Title: (1013–1032) Healthcare Disparities in Rheumatology Poster II: Socioeconomic Determinants
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Janus kinase inhibitors (JAKi) have therapeutic benefits in autoimmune conditions. Access to these medications is controlled by insurance carriers through requirements for prior authorizations and the use of restricted formularies which are difficult to navigate and may lead to denials of coverage and/or delays in dispensation.This study sought to characterize the real-word coverage denials and delays in dispensation of JAKi in patients with rheumatologic diseases, hypothesizing that there would be differences in denials of prescriptions and delays in dispensation related to drug type, race, and insurance status.
Methods: A retrospective cohort was created from patients at an academic tertiary care rheumatology practice who were prescribed a JAKi between 2014 and 2023. Patients were included in this analysis if they had a documented visit with a rheumatologist documented in the electronic health record (EHR) and for the initial prescription of a JAKi (tofacitinib, baricitinib, upadacitinib) but did not include patients renewing an existing prescription. Patients were selected irrespective of underlying disease. Demographics and current/prior disease-modifying anti-rheumatic drug (DMARD) use were extracted from the EHR. To provide confirmation of insurance approval and determine the time to dispensation, pharmacy and nursing-based communications related to the processing of prior authorization and coordination with specialty pharmacies were also extracted from the EHR.
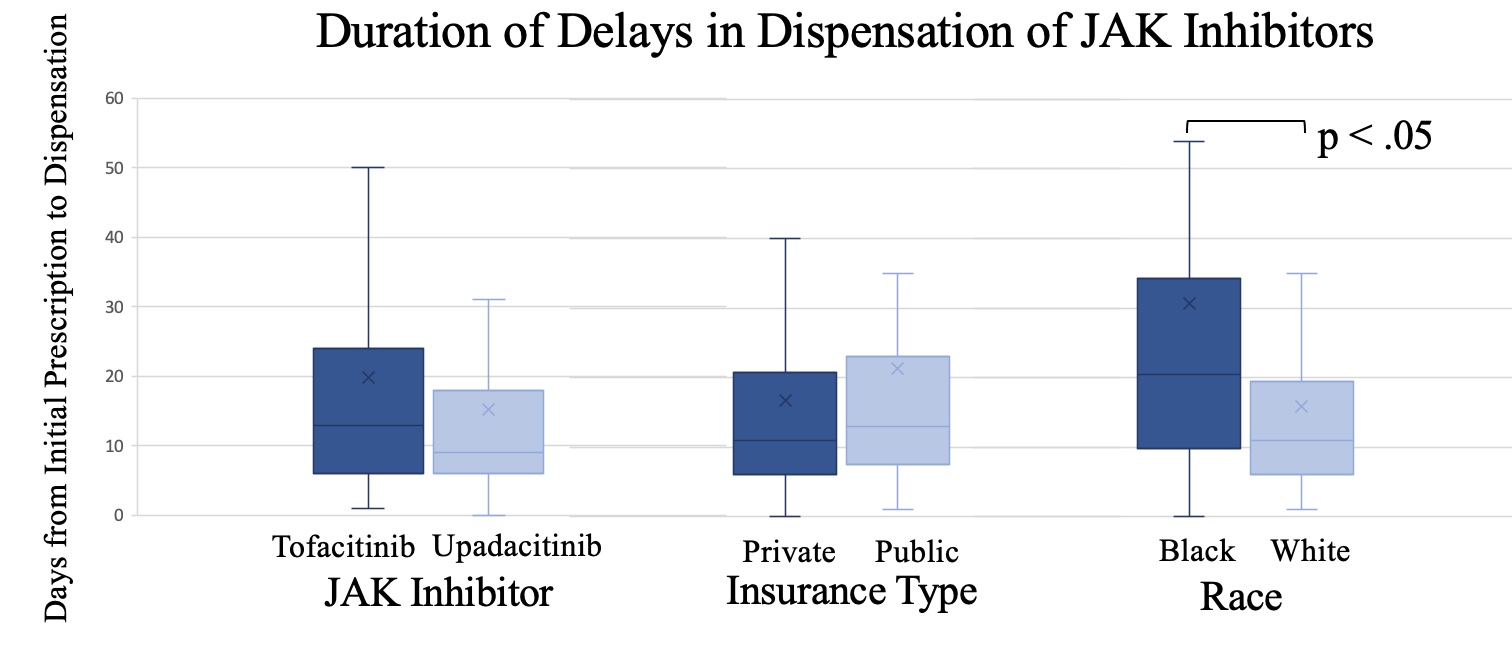
Results: We identified a total of 118 new prescriptions for JAKi among 90 patients (81 tofacitinib, 36 upadacitinib, 1 baricitinib).Of these 118 prescriptions, 10 (8%) had coverage denied by insurance, and 5 (4%) were not dispensed for other reasons [2 were limited by co-pay, 1 changed insurance, 1 developed a severe infection, and 1 opted not to start due to personal preferences]. Patients with an approved prescription experienced, on average, an 18-day delay [IQR 6-22] between initial prescription and dispensing of a JAKi. 14 prescriptions (11%) had delays longer than 30 days. Patients who were Black experienced a longer delay in initial dispensing compared to those who were Non-Hispanic White (30.6 vs 15.8, p = 0.013). Comparing private vs public insurance, there were not differences in rates of coverage denial (10% vs 7%, p = 0.61), nor were there differences in delays in dispensation (21.3 vs 16.7, p = 0.32). There were not differences in delays in dispensation for tofactinib compared to upadacitinib (19.8 vs 15.2, p =0.34).
Conclusion: While rates of coverage denial of JAKi were low, there was an average delay in dispensing prescriptions of 18 days. Patients who were Black experienced an almost 2-fold longer delay in dispensing of JAKi compared to those who identified as Non-Hispanic White. Further evaluation is needed to identify the potential factors at play contributing to this observed disparity and the key drivers of delays in medication dispensing.
To cite this abstract in AMA style:
Riley T, Dombrovsky I, George M, Baker J. Patterns in the Prescription, the Denials of Coverage, and the Delays in Dispensation of Janus Kinase Inhibitors [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/patterns-in-the-prescription-the-denials-of-coverage-and-the-delays-in-dispensation-of-janus-kinase-inhibitors/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-in-the-prescription-the-denials-of-coverage-and-the-delays-in-dispensation-of-janus-kinase-inhibitors/