Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
The management of patients with rheumatic diseases is partly guided by the medical history at each clinic visit. Patients however often find it difficult to accurately remember the course of their symptoms between appointments. Regular App-based patients’ self-monitoring of disease between clinic visits might provide an innovative and feasible improvement. The COmPASS I study [1] demonstrated that RA patients’ self-assessments of disease activity via App correlate strongly with clinicians’ assessments.
The main aims of COmPASS II are to assess if continuous self-monitoring of the disease optimises disease management in rheumatic diseases, and to assess the fluctuation of disease activity between clinic visits. This abstract describes the set-up and recruitment of the COmPASS II study in the first 16 months.
Methods:
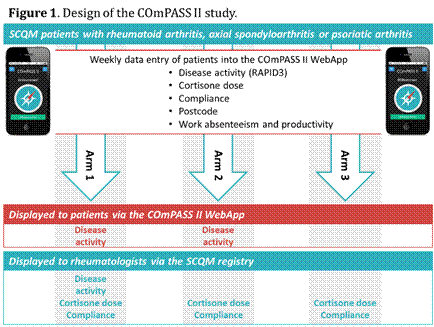
The COmPASS II App questionnaire consists of the RAPID3 score, a validated, commonly used PRO to self-assess disease activity. Additionally, patients are asked about their therapy compliance and cortisone dose. COmPASS II is embedded in the SCQM (Swiss Clinical Quality Management for Rheumatic Diseases) registry and hence allows the linkage of data obtained via the WebApp from the patients with routine clinical data collected in the SCQM. Interested SCQM patients with RA, axSpA or PsA are electronically randomized into 3 study arms (Figure). In arm 1 patients and rheumatologists are displayed the self-assessed disease activity over time, the patient directly via the App and the rheumatologists via SCQM. In arm 2 only patients are displayed their chart and in study arm 3 neither sees the recorded data. Patients are encouraged to fill in the App weekly.
Results:
The COmPASS II App went online in 02/2016. In the first 16 months, 329 patients were enrolled. 65% of patients used the App, 78% of those filled in the questionnaires for longer than a month; currently, the longest follow-up is 16 months. On average patients use the App every 2 weeks.
Patients using the App for longer than 6 months rated the user-friendliness/usability of the WebApp as very positive in terms of the ease of use (mean of 82 on the system usability scale, a score of 68 is considered average [2]) and indicated that they felt a benefit in terms of patient physician communication with a mean score of 58 on the 100-mm VAS.
Conclusion:
Patients are highly adherent in the App use, rate it as very user-friendly and feel a benefit in patient physician communication. The COmPASS II study will validate the utility of app-based patients’ self-assessments in enhancing disease control.
References:
1 Walker UA, et al. Disease activity dynamics in RA: patients’ self-assessment of disease activity via WebApp. Rheumatol 2017; in press.
2 Sauro J. Measuring Usability with the System Usability Scale. 2011. http://www.measuringu.com/sus.php
Acknowledgements: COmPASS II is supported by an unrestricted grant from AbbVie.
To cite this abstract in AMA style:
Jaeger VK, Barmet A, Schiffer P, Zufferey P, Badaracco A, Walder M, Dudler J, Frey D, Müller F, Pichler L, Voss P, Bosia L, Walker UA. Patient’s Self-Monitoring of Disease Activity of Rheumatic Diseases Via Webapp – Study Design, Patient’s Perspective and Recruitment in the First 16 Months of a Swiss Multicentre, Longitudinal Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/patients-self-monitoring-of-disease-activity-of-rheumatic-diseases-via-webapp-study-design-patients-perspective-and-recruitment-in-the-first-16-months-of-a-swiss-multicent/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patients-self-monitoring-of-disease-activity-of-rheumatic-diseases-via-webapp-study-design-patients-perspective-and-recruitment-in-the-first-16-months-of-a-swiss-multicent/