Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose:
Systemic lupus (SLE) may have a significant impact on multiple dimensions of quality of life. Patient-reported outcome (PRO) tools may provide valuable insights for clinical trials and treatment decisions in clinical practice. LupusPRO is a disease targeted, patient reported outcome measure designed specifically for patients with SLE. It includes 43 variables of which 30 items are health-related. The SF-36 Health Survey is a widely used, multipurpose, short-form index comprised of 36 items suitable for assessment of all medical conditions. Both instruments are internationally validated and have been correlated to each other in many lupus studies. The aim of this study was to compare SF-36 and LupusPRO scores in a racially and culturally diverse cohort of SLE patients.
Methods: The Cohort for Rheumatic Diseases is a longitudinal real-world study that follows 1,635 patients, including an embedded Lupus Cohort (674 patients who meet ACR classification criteria for SLE). 365 SLE patients completed the LupusPRO questionnaire and SF-36 during the same routine clinical visits between 2011 and 2019. Spearman’s rank tests and Kruskal-Wallis methods were used to compare age, race, gender, and year of disease to SF-36 and LupusPRO scores. Linear regression methods were then used to compare LupusPRO and SF-36 scores controlling for these variables.
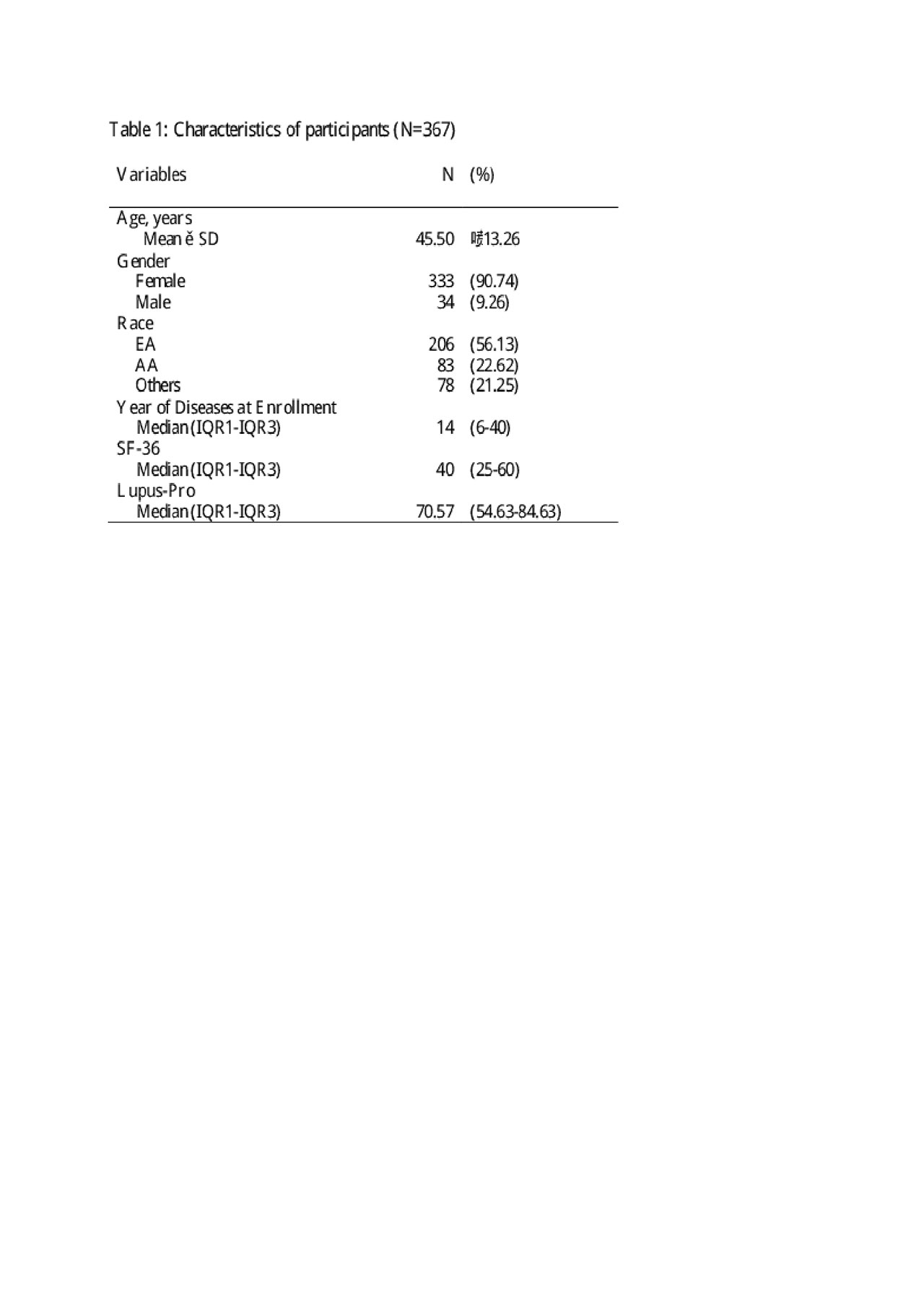
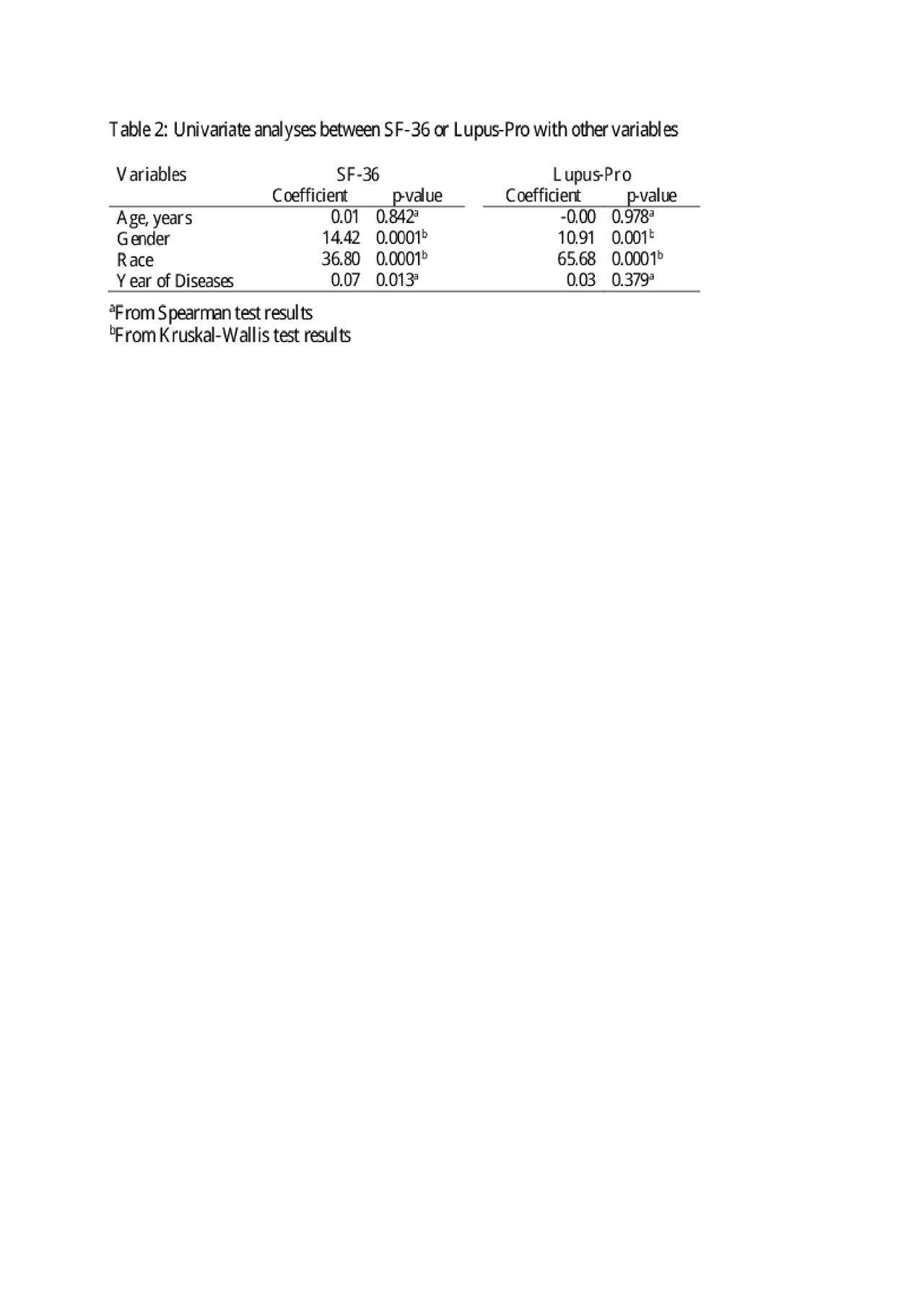
Results: The patients completed a mean of 3.43 paired results. Subjects were 90% female with a mean age of 45.50 (SD=13.26) years. The population was 56.1% Caucasian, 22.6% African American, and 21.3% Other Race, primarily consisting of American Indian and mixed race. In univariate analysis, results of the PROs were comparable (p< 0.0001) (Table 2) and African American patients had higher scores than European Americans (p=0.001) (Table 3). The positive correlation between LupusPRO and SF-36 remained in multivariate analysis (p< 0.0001) when controlled for race, gender, and year of disease (Table 3).
Conclusion: SF-36 and LupusPRO gave similar results. African American patients have higher scores on both instruments, indicating their report of better functioning and quality of life, despite their known increased disease severity. Both disease-targeted LupusPRO and generic SF-36 tools may be useful for measuring quality of life in SLE studies, but potential cultural and disease impacts on these measures need to be carefully considered
To cite this abstract in AMA style:
Zuech K, Tran L, Aberle T, Arriens C, Chakravarty E, Merrill J, James J. Patients of African Descent Score Higher on Quality of Life Indices Despite Their Known Disease Severity [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/patients-of-african-descent-score-higher-on-quality-of-life-indices-despite-their-known-disease-severity/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patients-of-african-descent-score-higher-on-quality-of-life-indices-despite-their-known-disease-severity/