Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: To evaluate treatment outcomes based on the performance of a preliminary data-driven definition (PD) of a positive MRI of the SIJ in comparison to the established ASAS definition (ED) indicating eligibility into a clinical trial.
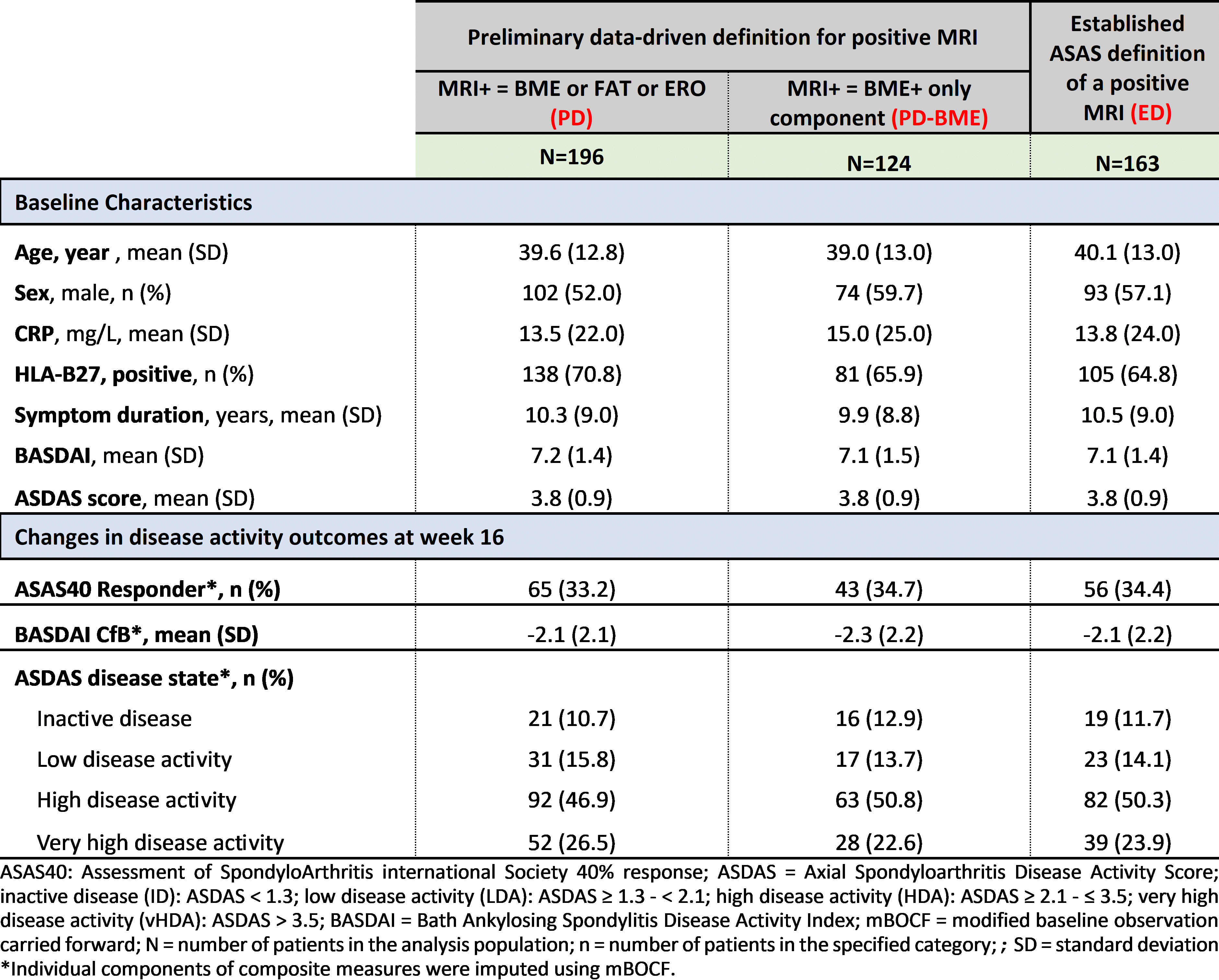
Methods: COAST-X is a phase 3, multicentre, randomized, double-blind, placebo-controlled study in nr-axSpA patients, receiving 80 mg ixekizumab every 2 or 4 weeks (wk), or placebo up to wk52. Baseline SIJ MRIs were scored by two central readers. Treatment arms were pooled for these descriptive analyses. The per protocol population was categorised using the total PD, PD-BME component, and the established ASAS definition (ED) of a positive SIJ MRI (Table 1). Baseline characteristics and the percentage of patients achieving ASDAS disease states, ASAS40 response and mean BASDAI change from baseline (CfB) are reported at wk16 based on each definition. Modified baseline observation carried forward (mBOCF) were used for missing data.
Results: At baseline, N=263 patients, the mean age was 39.6, 39.0 and 40.1, and percentage of males was 52.0%, 59.7% and 57.1% for PD (total), PD-BME and ED, respectively. Mean BASDAI and ASDAS were similar for PD, PD-BME and ED. 75% (196/263), 47% (124/263) and 62% (163/263) of patients were deemed MRI+ using PD, PD-BME and ED, respectively. At wk16, similar proportion of patients achieved ASAS40 in PD, PD-BME and ED (33.2%, 34.7% and 34.4%) and similar BASDAI CfB (-2.1, -2.3 and -2.1). The proportion of patients achieving an ASDAS inactive disease (10.7%, 12.9% vs 11.7%) or low disease activity (15.8%, 13.7% vs 14.1%) at wk16, was similar for PD, PD-BME and ED (Table 2).
Conclusion: Patients categorised using PD vs ED of a positive MRI, showed similar baseline characteristics and clinical outcomes after 16wks. The PD did not outperform the ED in the specificity of patient selection for a clinical trial in nr-axSpA.
To cite this abstract in AMA style:
Baraliakos X, Navarro Compán V, Machado P, Bolce R, Russ H, Ngantcha M, Lisse J, Jing Ng K, Ramiro S, Poddubnyy D. Patient Selection and Treatment Outcomes Using Preliminary Data-driven Definition versus the Established ASAS Definition of a Positive MRI of the Sacroiliac Joint in axSpA: Post-hoc Analysis from COAST-X [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/patient-selection-and-treatment-outcomes-using-preliminary-data-driven-definition-versus-the-established-asas-definition-of-a-positive-mri-of-the-sacroiliac-joint-in-axspa-post-hoc-analysis-from-coas/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-selection-and-treatment-outcomes-using-preliminary-data-driven-definition-versus-the-established-asas-definition-of-a-positive-mri-of-the-sacroiliac-joint-in-axspa-post-hoc-analysis-from-coas/