Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient Reported Outcomes (PROs) provide important information on assessment of pain, disease activity, fatigue and physical function in patients with psoriatic arthritis (PsA). Knowledge of the evolution in PROs over time in patients, who initiate treatment with their 1st, 2nd or 3rd Tumour Necrosis Factor inhibitor (TNFi) in routine practice is limited. Hence, the aim of this was study was to investigate PROs at 6, 12 and 24 months after TNFi start and the changes from baseline to 6, 12 and 24 months in PsA patients, who initiated their 1st, 2nd or 3rd TNFi in clinical practice.
Methods: Pooled data on PsA patients from 13 European registries participating in the EuroSpA Research Collaboration were analysed (1). Patients were included in the study cohort if they had been followed in the registry from initiation of the 1st TNFi. PROs included Visual Analogue Scale (VAS) scores on a 0-100 mm scale for pain, global disease activity and fatigue, while physical function was captured with Health Assessment Questionnaire (HAQ). The distribution of PROs at 6, 12 and 24 months after TNFi start and the changes from baseline to 6, 12 and 24 months were investigated with descriptive statistics. PRO remission rates were defined as the proportion of patients achieving a state of pain score ≤20 mm, global score 20 mm, fatigue score ≤20 mm and HAQ score ≤0.5. Crude and LUNDEX-adjusted (2) PRO remission rates were assessed for the overall cohort and the individual registries.
Results: Of the 25,988 axSpA patients, who initiated 1st TNFi, 8,294 patients switched to a 2nd TNFi, while 2,842 patients subsequently switched to a 3rd TNFi. Baseline characteristics of the study cohort are shown in Table 1. The 6, 12 and 24 month PRO status and changes in PROs for 1st, 2nd and 3rd TNFi in the pooled cohort are summarized in Table 2. For the 1st, 2nd and 3rd TNFi, median PROs after 6 months ranged from 20mm to 30mm, 27mm to 40mm and 35mm to 50mm, respectively. Similarly, median decreases in PROs from baseline to 6 months ranged from 18 to 30mm, 8 to 18mm and 8 to 18mm for the 1st, 2nd and 3rd TNFi. In the overall cohort 6 month LUNDEX-adjusted PRO remission rates varied from 33% to 41%, 23% to 33% and 17% to 24% for the 1st, 2nd and 3rd TNFi, respectively (Table 2). In the individual registries, LUNDEX-adjusted 6 months PRO remission rates for the 1st TNFi ranged from 32% to 51%, 24% to 50%, 28% to 48%, 32% to 51% and 21% to 47% for pain, global, BASDAI, BASFI and fatigue scores, respectively.
Conclusion: In this large observational study cohort, one-third of patients achieved a state of PRO remission after 6 months of treatment with their first TNFi with significant variation between registries. As expected, improvements in PROs and PRO remission rates were lower in those who had switched to the 2nd or 3rd TNFi, reflecting selection of non-responders and more severe cases (confounding by indication).
References:
1 Brahe et al. Arthritis Rheum, 2018; 70 (suppl 10)
- Kristensen et al. Arthritis Rheum, 2006, 54(2), p:600-6
Acknowledgements: Novartis Pharma AG and IQVIA for supporting the EuroSpA
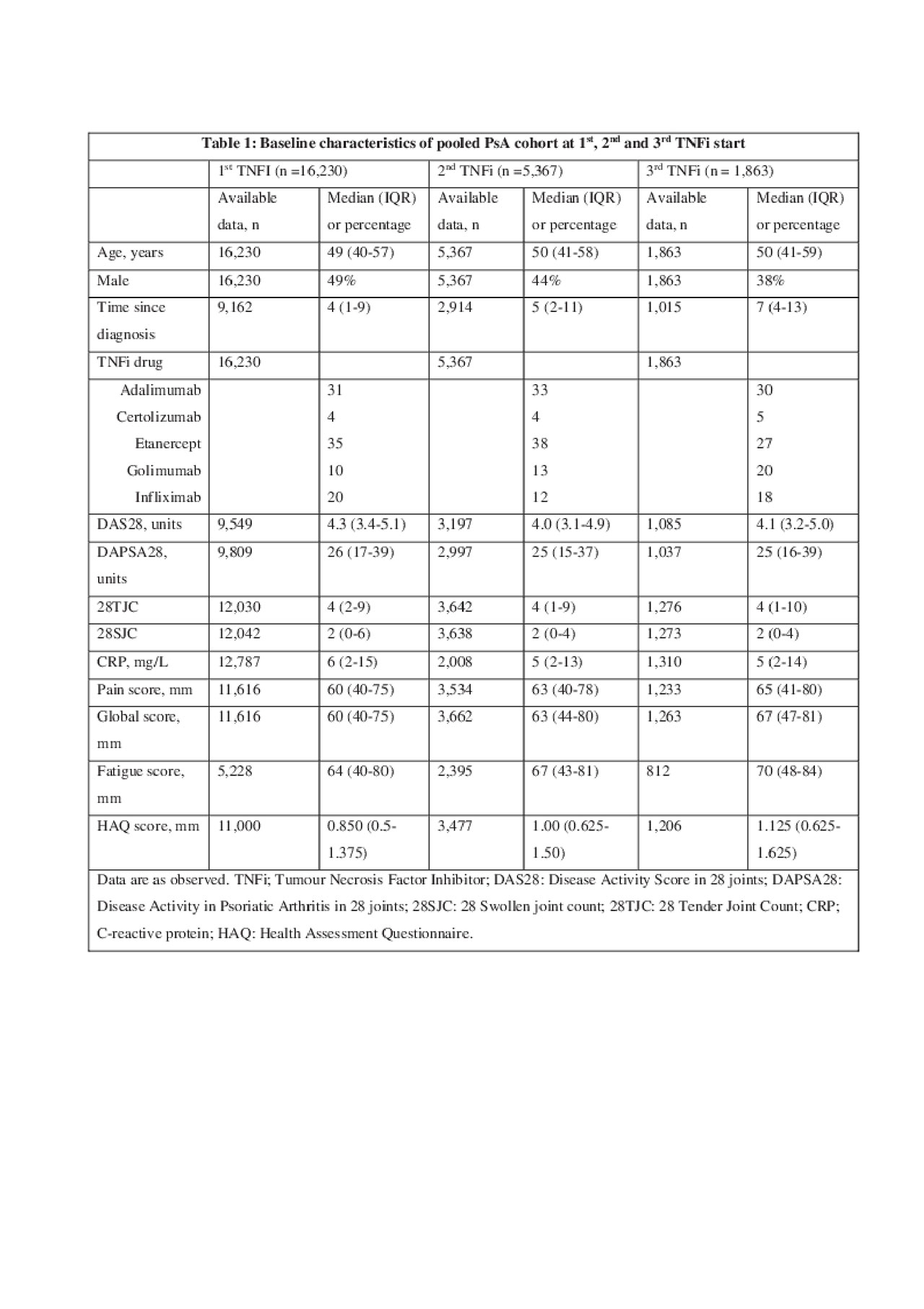
20190603_EuroSpA_study2.1_table1
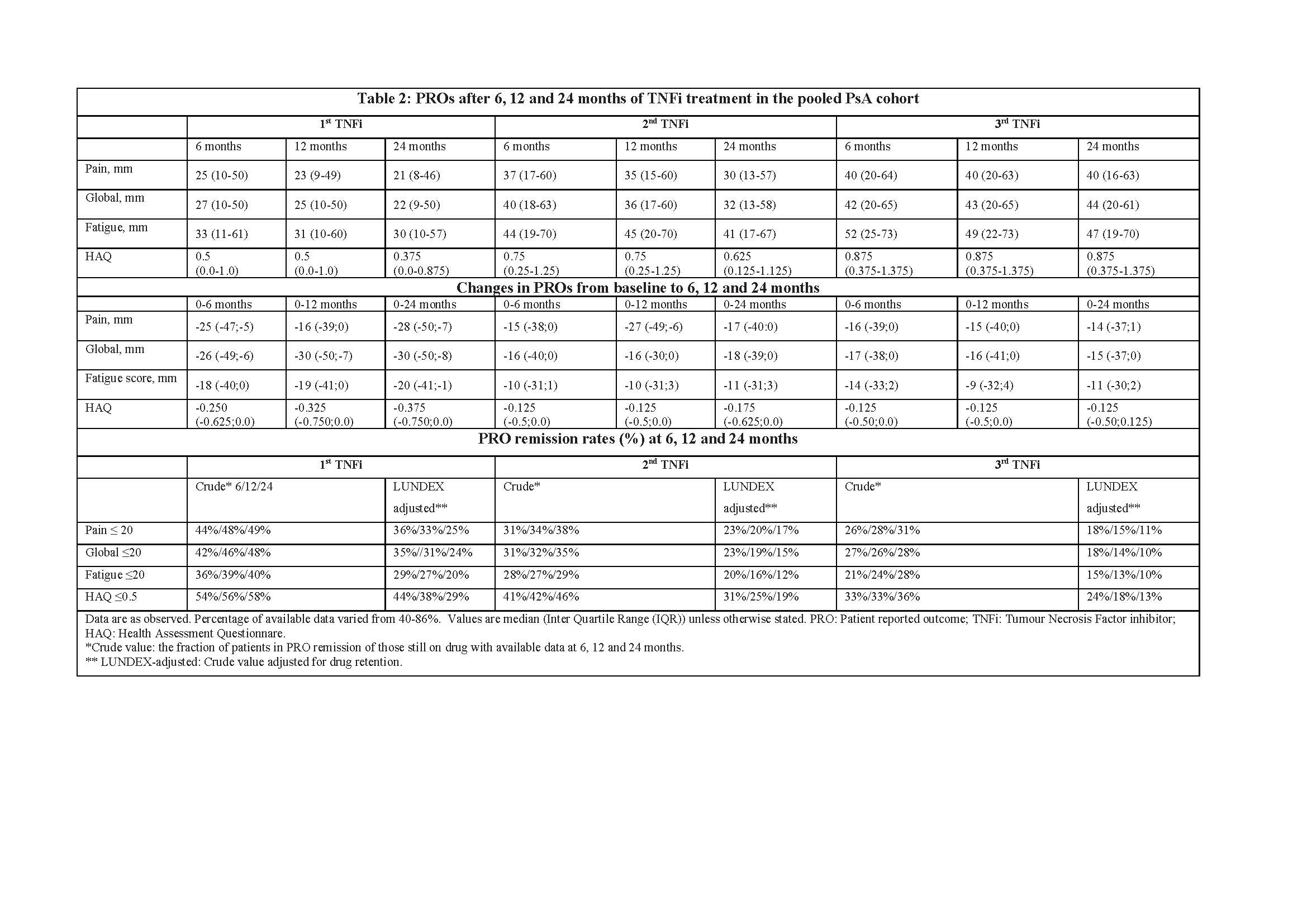
20190603_EuroSpA_study2.1_table2
To cite this abstract in AMA style:
Ørnbjerg L, Jacobsson L, Loft A, Iannone F, Nissen M, Kristianslund E, Mann H, Santos M, Pombo-Suarez M, Eklund K, Rotar Z, Gudbjornsson B, Öztürk M, Codreanu C, van de Sande M, Wallman J, Favalli E, Moeller B, Sexton J, Pavelka K, Vieira-Sousa E, Sánchez-Piedra C, Trokovic N, Tomsic M, Love T, Cefle A, IONESCU R, van der Horst-Bruinsma I, Jones G, Heegaard Brahe C, Lund Hetland M, Østergaard M. Patient Reported Outcomes over 2 Years in Psoriatic Arthritis Patients Initiating Treatment with 1st, 2nd or 3rd TNF Inhibitor in Routine Care – Was PRO Remission Achieved? Results from the EuroSpA Collaboration [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/patient-reported-outcomes-over-2-years-in-psoriatic-arthritis-patients-initiating-treatment-with-1st-2nd-or-3rd-tnf-inhibitor-in-routine-care-was-pro-remission-achieved-results-from-the-eu/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-over-2-years-in-psoriatic-arthritis-patients-initiating-treatment-with-1st-2nd-or-3rd-tnf-inhibitor-in-routine-care-was-pro-remission-achieved-results-from-the-eu/
