Session Information
Date: Sunday, November 10, 2019
Title: 3S076: Patient Outcomes, Preferences, & Attitudes I: Patient Reported Outcomes (833–838)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Therapeutic options for patients with PsA are increasing but data directly comparing biologics are limited. The multicentre, open-label SPIRIT-H2H trial (NCT03151551) evaluated the efficacy and safety of ixekizumab (IXE) vs adalimumab (ADA) over a 52-week treatment period in biologic DMARD-naïve patients with active PsA. This analysis directly compared the effects of IXE vs ADA on patient-reported outcomes (PROs) and health-related quality of life (HRQoL) after 24 weeks’ treatment in SPIRIT-H2H.
Methods: All patients fulfilled the Classification Criteria for Psoriatic Arthritis (CASPAR). Patients were randomised (1:1) to 52 weeks of IXE (160 mg week 0, then 80 mg every 2 weeks [Q2W] to week 12 and Q4W thereafter for patients with moderate-to-severe plaque psoriasis [PsO] or 160 mg week 0, then 80 mg Q4W for other patients) or ADA (80 mg week 0 then 40 mg Q2W from week 1 for patients with moderate-to-severe PsO or 40 mg week 0 then 40 mg Q2W for other patients). The primary objective was to assess the superiority of IXE vs ADA by comparing the proportion of patients achieving a simultaneous improvement of ≥50% in ACR criteria (ACR50) and 100% in Psoriasis Area Severity Index score (PASI100) at week 24. A number of PROs and HRQoL were evaluated (see Table). PRO changes from baseline to week 24 were analysed using analysis of covariance models with modified baseline observation carried forward imputation. Treatment satisfaction was assessed using the Treatment Satisfaction Questionnaire. Logistic regressions with non-responder imputation in the intent-to-treat population were used to evaluate results.
Results: A total of 566 patients were randomised to IXE or ADA; of these 82% of patients did not have moderate-to-severe PsO. Baseline characteristics were generally balanced between the two treatment groups. The percentage of patients simultaneously achieving ACR50 and PASI100 responses at week 24 was statistically superior for IXE vs ADA (36% vs 28%; p< 0.05). All PRO and HRQoL outcomes (Table) significantly improved from baseline in both treatment groups (p< .001 for all). Treatment with IXE was associated with a statistically significantly greater improvement in itch, Short form-36 Health Survey scores, Role-emotional and Dermatology Life Quality Index (DLQI) scores vs ADA (seen as early as week 4 for itch and DLQI improvement). For patients’ overall satisfaction with treatment, 62.5% of IXE and 59.7% of ADA recipients were mostly satisfied. Both biologics had acceptable safety and tolerability.
Conclusion: In bDMARD-naïve patients with active PsA and PsO, IXE showed superior efficacy compared to ADA based on simultaneous achievement of ACR50 and PASI100 responses at week 24. Both biologics significantly improved HRQoL and other PROs. Greater improvements in PROs related to PsO were observed for IXE- compared to ADA-treated patients.
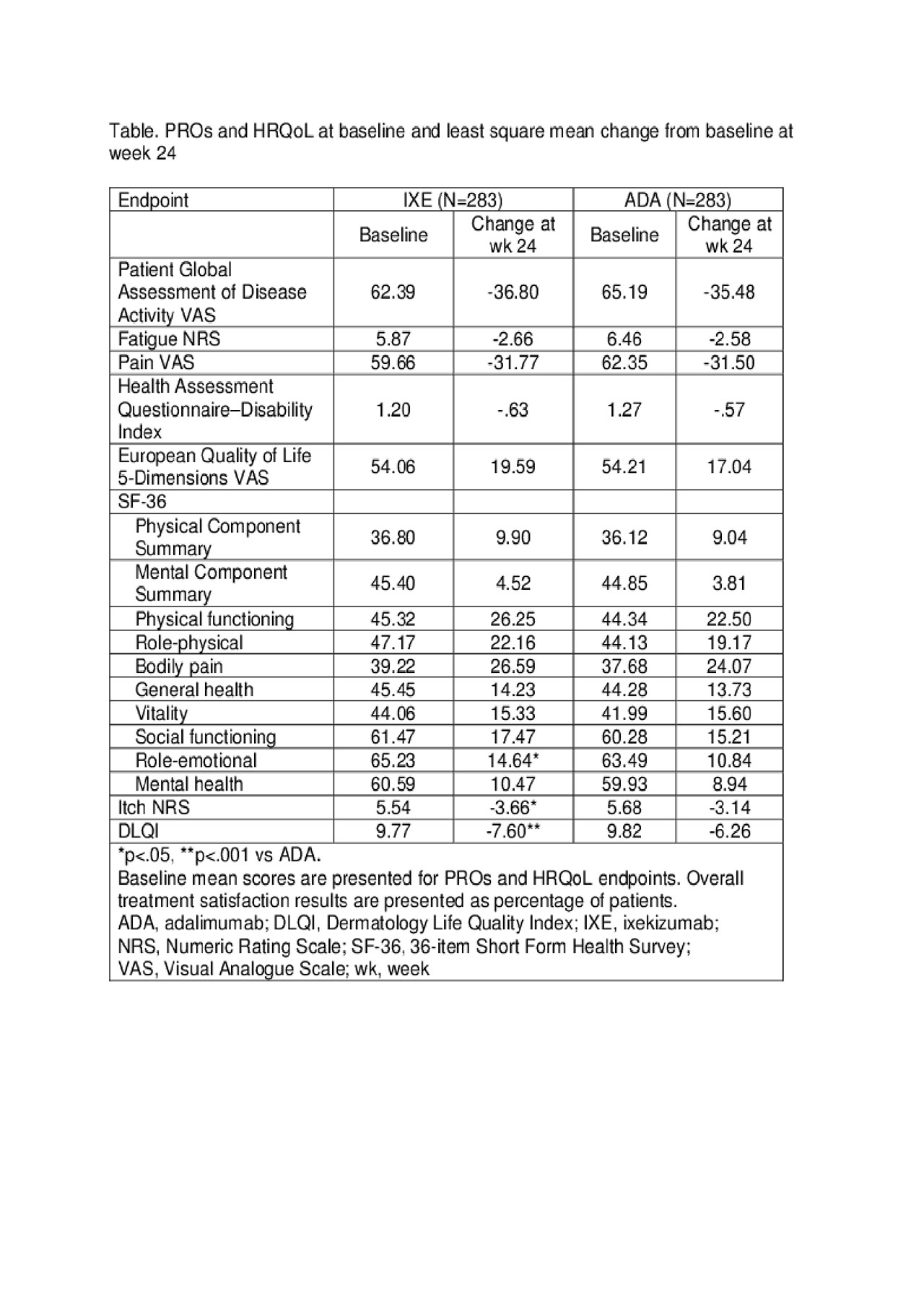
Table. PROs and HRQoL at baseline and least square mean change from baseline at week 24
To cite this abstract in AMA style:
Van den Bosch F, Kristensen L, McGonagle D, Rossini M, Liu-Leage S, Sapin C, Meszaros G, Merola J. Patient-Reported Outcomes from a Randomised, Open-Label, Parallel-Group Study Evaluating Ixekizumab versus Adalimumab in Patients with PsA Who Are Biologic DMARD Naïve: 24-Week Results [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/patient-reported-outcomes-from-a-randomised-open-label-parallel-group-study-evaluating-ixekizumab-versus-adalimumab-in-patients-with-psa-who-are-biologic-dmard-naive-24-week-results/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-from-a-randomised-open-label-parallel-group-study-evaluating-ixekizumab-versus-adalimumab-in-patients-with-psa-who-are-biologic-dmard-naive-24-week-results/
