Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
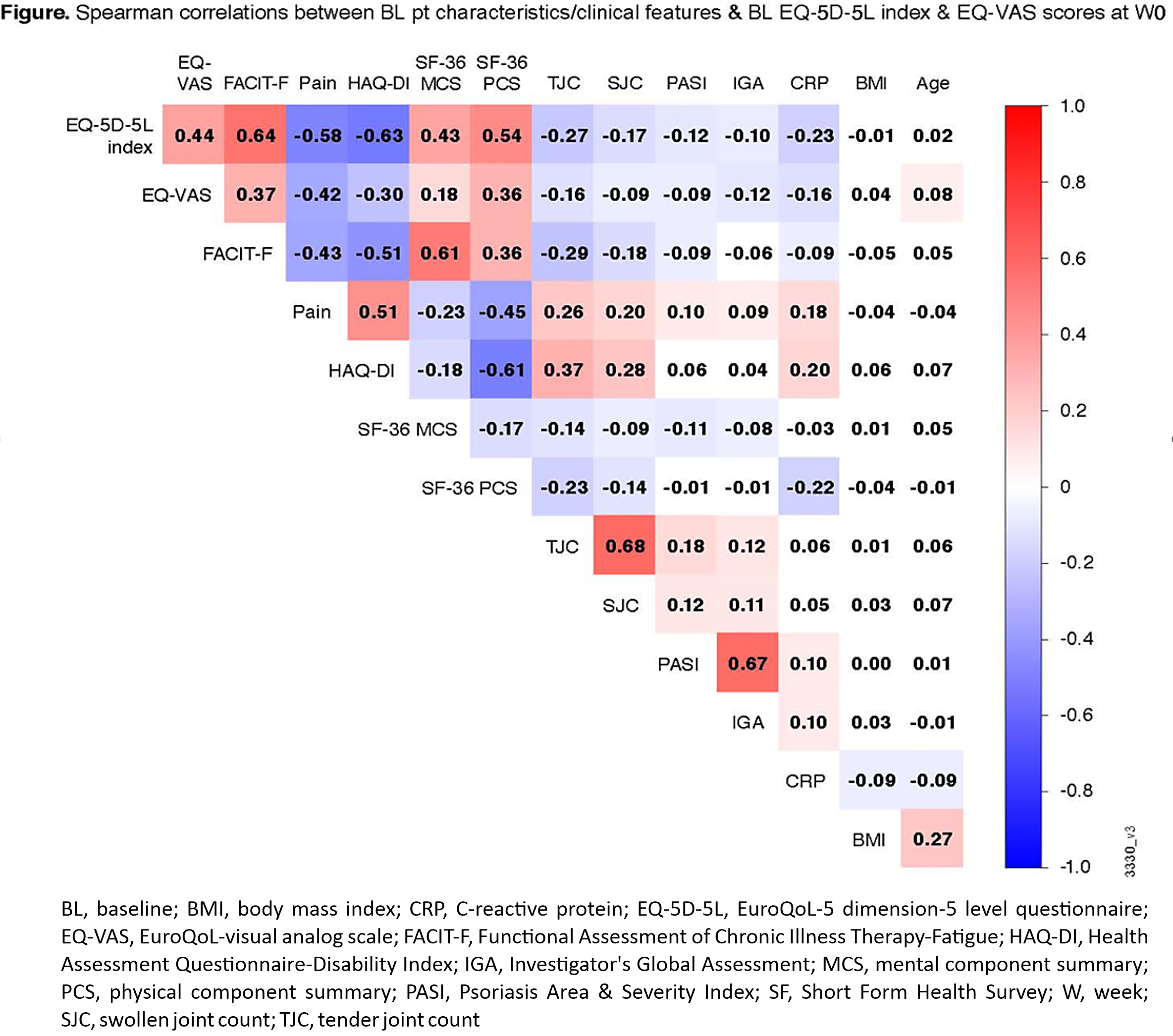
Background/Purpose: PsA is a chronic inflammatory disease characterized by peripheral arthritis, axial inflammation, dactylitis, enthesitis, & skin/nail psoriasis. Patients (pts) with PsA often experience reduced health-related quality of life (HRQoL) due to these features. Using EuroQoL-5 dimension-5 level (EQ-5D-5L) questionnaire index & visual analog scale (EQ-VAS) scores, we assessed HRQoL in PsA pts & its association with pt characteristics & clinical features of PsA, including fatigue.
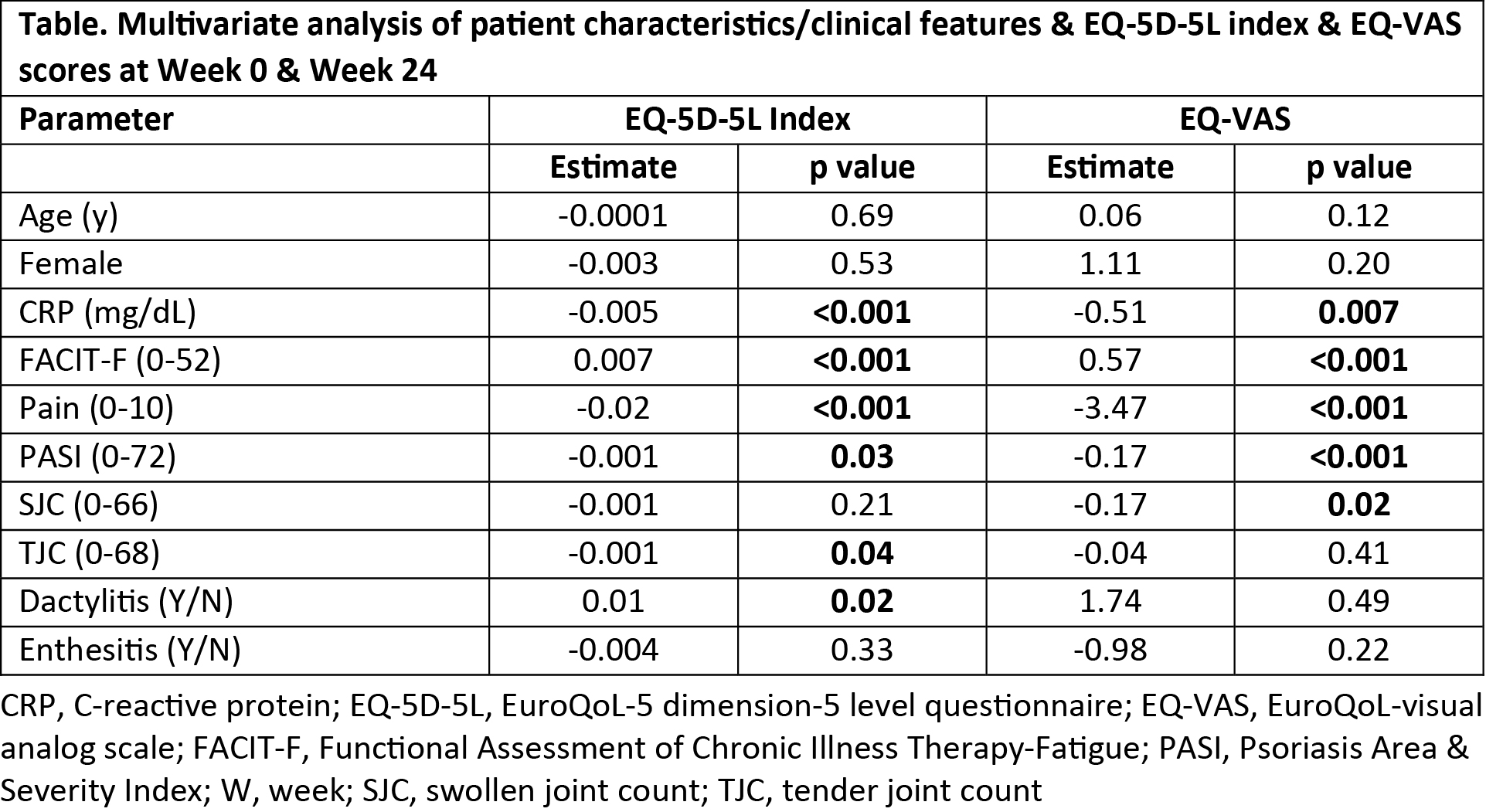
Methods: The phase 3 DISCOVER-2 trial evaluated guselkumab (GUS), an anti-IL-23p19-subunit mAb, in bio-naïve adults with active PsA (swollen joint count [SJC] ≥5, tender joint count [TJC] ≥5, CRP ≥0.6 mg/dL) despite standard therapies.1 Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at Week (W) 0, W4, then Q8W; or placebo (PBO). EQ-5D-5L index assesses mobility, self-care, usual activities, pain/discomfort, & anxiety/depression. EQ-VAS assesses pt health state. Spearman correlation testing was used to evaluate relationships between baseline (BL) pt characteristics & PsA clinical features & BL EQ-5D-5L index & EQ-VAS scores (Fig). Employing absolute observed scores at W0 & W24, univariate linear regression was used to assess the association between EQ-5D-5L index & EQ-VAS scores & pt characteristics/PsA clinical features. Variables with p< 0.20 in the univariate analysis were included in a multivariate analysis employing mixed-effect model for repeated measures (MMRM), controlling for all other variables; p< 0.05 was considered statistically significant. Least-squares (LS) mean changes in EQ-5D-5L index & EQ-VAS were assessed at W24 using MMRM.
Results: Among 738 pts, BL EQ-5D-5L index & EQ-VAS scores were moderately to strongly correlated (≥0.4) with BL pt-reported pain (0-10 VAS), physical function (HAQ-Disability Index [DI]), fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-F] scale), & 36-item Short Form Health Survey (SF-36) physical & mental component summary (PCS & MCS) scores; & weakly correlated with other variables (Fig). Based on univariate analyses (p< 0.20) & evaluation of collinearity between variables, attributes at W0 & W24 included in the multivariate models were age, sex, CRP, FACIT‑F, pain, Psoriasis Area & Severity Index (PASI) score, TJC, SJC, enthesitis, & dactylitis. In the final model, CRP, FACIT-F, pain, PASI score, & the presence of dactylitis were significantly associated with EQ-5D-5L index & EQ-VAS scores. A higher TJC was significantly associated with a worse EQ-5D-5L index score. A higher SJC was significantly associated with a worse EQ-VAS score (Table). For reference, in the GUS Q4W (N=244), GUS Q8W (N=246), & PBO (N=244) groups, LS mean changes from BL at W24 were 0.12, 0.12, & 0.05, respectively, for EQ-5D-5L index & 18.1, 18.4, & 6.8, respectively, for EQ-VAS.
Conclusion: Joint & skin symptoms, dactylitis, fatigue, pain, & elevated CRP levels were significantly associated with reduced HRQoL (measured by EQ-5D-5L index & EQ-VAS) in bio-naïve pts with active PsA. Treatment of multiple PsA domains may help optimize HRQoL. Improvement across clinical domains1 & in HRQoL was observed in GUS-treated PsA pts.
1. Mease P, et al. Lancet 2020;395:1126-36.
To cite this abstract in AMA style:
Curtis J, McInnes I, Rahman P, Gladman D, Yang F, Peterson S, Agarwal P, Kollmeier A, Hsia E, Han C, Shawi M, Tillett W, Mease P. Patient Characteristics and Clinical Features Associate with Health-Related Quality of Life in Bio-naïve Patients with Active Psoriatic Arthritis Through Week 24 of the DISCOVER-2 Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/patient-characteristics-and-clinical-features-associate-with-health-related-quality-of-life-in-bio-naive-patients-with-active-psoriatic-arthritis-through-week-24-of-the-discover-2-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-characteristics-and-clinical-features-associate-with-health-related-quality-of-life-in-bio-naive-patients-with-active-psoriatic-arthritis-through-week-24-of-the-discover-2-study/