Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Sleep quality is diminished in patients with psoriatic arthritis (PsA) and close to 40% of PsA patients consider sleep impairment a priority domain.This work analyzed determinants of impaired sleep in patients with PsA.
Methods: This was a cross-sectional analysis of an observational study (ReFlap, NCT03119805), which included adult patients with definite PsA with ≥ 2 years disease duration from 14 countries (ref 1). Sleep was assessed using the patient self-reported evaluation of sleep on a 0-10 numerical scale, included in the Psoriatic Arthritis Impact of Disease questionnaire (PSAID-12) (ref 2). A score ≥ 4 was considered as sleep impairment. Demographic and clinical variables associated to sleep impairment were assessed through univariate analysis; prevalence ratios (PR)[95% CI] were reported. Variables independently associated to sleep impairment in the univariate analysis (with p-value < 0.05) were entered in the Poisson regression model.
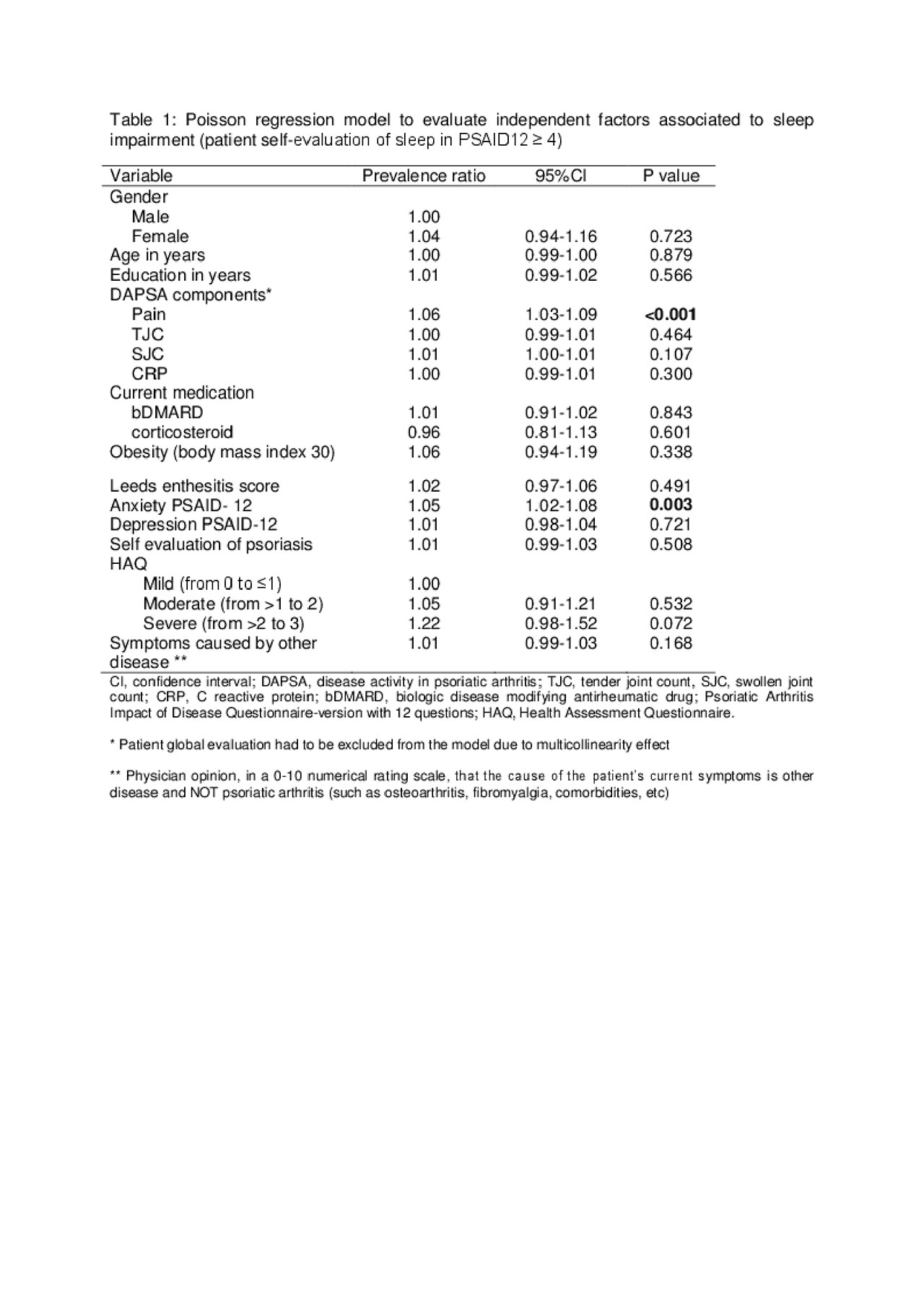
Results: A total of 396 patients were analyzed: mean age 51.9 ± 12.6, 51% (N= 202) were females, 59.7% were receiving biologic therapy (N= 221), 53.3% (N=201) of participants had 1-5% of body surface area (BSA) affected by psoriasis, 74% (N=293) had mild disability (defined as a score ≤1 in the Health Assessment Questionnaire-HAQ); 23.7% (N=94) were in remission and 36.9% (N=146) in low disease activity according to the Disease Activity in Psoriatic Arthritis (DAPSA) score. Median (25th-75th) patient’s self-evaluation of sleep difficulties was 2 (0-6), 39.9% (N=158) were considered as having sleep impairment. In the univariate analysis, factors independently associated to sleep impairment were: moderate (HAQ >1 to 2) and severe (HAQ >2 to 3) disability (PR: 1.55 [1.39-1.72] and 2.07 [1.97-2.18] respectively, p< 0.001), glucocorticoid use (PR: 1.52 [1.16-1.99], p=0.003), obesity (PR: 1.50 [1.06-2.11], p=0.021), psoriasis affecting >20% of BSA (PR: 1.12 [1.08-1.17], p< 0.001), moderate and high disease activity by DAPSA (PR: 7.01 [3.54-13.8] and 8.71 [4.46-17.9] respectively, p< 0.001) and self-reported levels of anxiety, depression and fatigue (PR: 1.22 [1.18-1.28], 1.16 [1.14-1.21] and 1.28 [1.23-1.33] respectively, p< 0.001). Poisson regression model showed only self-reported levels of anxiety (PR: 1.05 [1.02-1.08], p=0.003) and the pain component of DAPSA (PR: 1.06 [1.01-1.09], p< 0.001) contributing to sleep impairment (Table 1).
Conclusion: Sleep impairment was frequent in this population of PsA patients; pain and anxiety were independently associated to sleep impairment.
To cite this abstract in AMA style:
Palominos P, Coates L, Kohem C, Orbai A, Smolen J, de Wit M, Kiltz U, Leung Y, Cañete J, Scrivo R, Balanescu A, Dernis E, Talli S, SOUBRIER M, Aydin S, Gaydukova I, Kalyoncu U, Gossec L. Pain and Anxiety Are Independent Factors Associated to Sleep Impairment in Psoriatic Arthritis: A Multicentric Study in 14 Countries [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pain-and-anxiety-are-independent-factors-associated-to-sleep-impairment-in-psoriatic-arthritis-a-multicentric-study-in-14-countries/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pain-and-anxiety-are-independent-factors-associated-to-sleep-impairment-in-psoriatic-arthritis-a-multicentric-study-in-14-countries/