Session Information
Session Type: ACR Late-breaking Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose:
No clinical trials have previously investigated the frequency of acute flares in early gout or the benefit of instituting urate-lowering therapy (ULT) earlier in the course of the disease. Currently, the standard of care for a vast majority of patients with early gout who have experienced only a single gout attack is limited to acute flare treatment with no ULT. In this analysis of data from a Phase 2 study, we have evaluated the frequency of acute flares in patients with early gout treated with febuxostat versus placebo during a 2-year period.Methods:
In this randomized, double-blind, placebo-controlled trial, patients with serum urate (sUA) ≥7.0 mg/dL and estimated glomerular filtration rate ≥60 mL/min were randomized to either febuxostat 40/80 mg or placebo for up to 2 years. Febuxostat was titrated from 40 to 80 mg at the Month 1 visit if sUA was ≥6.0 mg/dL at the Day 14 visit. All patients had early gout, defined as having experienced 2 or fewer gout flares in total and only 1 flare during the previous 12 months. Patients in both treatment groups received standard of care for acute gout flares throughout the study at the discretion of the investigator. All patients also received gout flare prophylaxis for the first 6 months of the study; patients who were either nonresponsive or intolerant to prophylaxis could be managed at the discretion of the investigator. The percentage of patients with at least 1 gout flare was summarized by time interval (Months 0–6, 6–12, 12–18, and 18–24) as well as for the overall study. Each percentage was calculated based on the number of patients who had at least 1 day of drug exposure in the corresponding time interval. Differences between treatment groups were assessed using Fisher’s exact test.Results:
A total of 314 patients received treatment with placebo (n=157) or febuxostat (n=157). During the first 6 months, the febuxostat group showed a slightly higher percentage of patients with at least 1 flare, which was not statistically significant (Figure). During the subsequent time intervals (Months 6–12, 12–18, and 18–24), the percentage of patients with at least 1 flare was consistently and significantly lower in the febuxostat group than the placebo group (Figure). Over the entire study duration, the percentage of patients with at least 1 flare was significantly lower in the febuxostat group than the placebo group (29.3% vs 41.4%, p<0.05).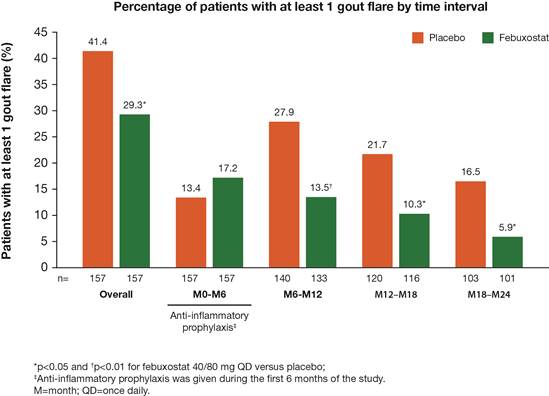
Conclusion:
Recurrent flares commonly occur in early gout, affecting >40% of patients over a 2-year period. This first clinical trial in patients with early gout demonstrates that treatment with febuxostat, to a target sUA of <6.0 mg/dL, can achieve a significant reduction in gout flares compared with placebo over a 2-year period, with significantly fewer gout flares during Months 6–12, 12–18, and 18–24.
Disclosure: N. Dalbeth, Ardea/AstraZeneca, 2,Ardea/AstraZeneca, Crealta, Cymabay, Takeda, 5; K. G. Saag, Ardea/AstraZeneca, Horizon, Takeda, 5,Ardea/AstraZeneca, Horizon, Takeda, 2; W. Palmer, None; H. K. Choi, AstraZeneca, 2,Takeda, 5; B. Hunt, Takeda, 3; P. MacDonald, Takeda, 3; U. Thienel, Takeda, 3; L. Gunawardhana, Takeda Pharmaceuticals, 3,Takeda Pharmaceuticals, 1.
To cite this abstract in AMA style:
Dalbeth N, Saag KG, Palmer W, Choi HK, Hunt B, MacDonald P, Thienel U, Gunawardhana L. Overall Reduction in Acute Flares during Treatment with Febuxostat Compared with Placebo over 2 Years in Patients with Early Gout [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/overall-reduction-in-acute-flares-during-treatment-with-febuxostat-compared-with-placebo-over-2-years-in-patients-with-early-gout/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/overall-reduction-in-acute-flares-during-treatment-with-febuxostat-compared-with-placebo-over-2-years-in-patients-with-early-gout/
