Session Information
Session Type: Poster Session A
Session Time: 6:00PM-7:00PM
Background/Purpose: Pediatric uveitis is commonly associated with rheumatic disease and can lead to sight-threatening complications if not properly treated. Systemic immunomodulatory therapy has dramatically changed prognosis of corticosteroid refractory uveitis. Autosomal dominant neovascular inflammatory vitreoretinopathy (ADNIV) is a rare autoimmune condition caused by mutations in CAPN5, typically diagnosed in adults, and characterized by intermediate uveitis, retinal degeneration and neovascularization. In the early stages it is asymptomatic but inevitably leads to permanent blindness despite treatment; however routine testing for CAPN5 and ophthalmic screening for uveitis is not regularly done in at-risk children. Proteomic studies have shown that IL6 and VEGF are elevated in the vitreous, suggesting a possible role for targeted therapy to alter disease trajectory. Our aim is to present the short-term outcomes of the first cohort of children diagnosed with ADNIV.
Methods: Cohort study of patients ≤18 years old at diagnosis with (+) CAPN5 gene mutation (p.Leu244Pro), ocular findings consistent with ADNIV and a minimum follow-up of 6months (m). Treatment response was defined as a decrease in 1) vitreous cells on clinical examination, 2) retinal vascular leakage on ultra-widefield fluorescein angiography (UWFA), and/or 3) macular edema on optical coherence tomography (OCT).
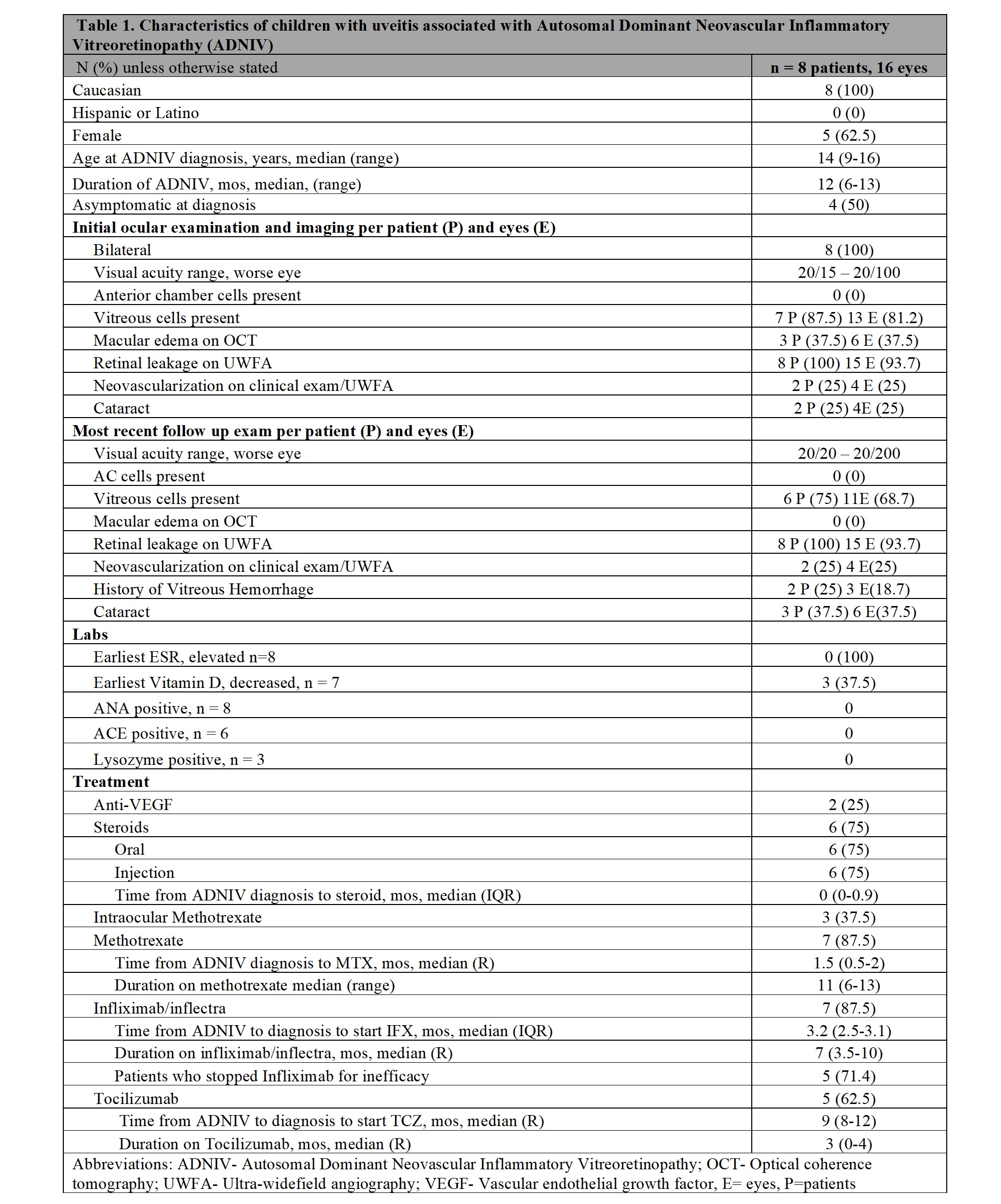
Results: Of 19 children with a family history of ADNIV, 9 were (+) for CAPN5 mutations and 8 (16 eyes) met the inclusion criteria (Table 1). Sixty-two percent were females, and median age of ADNIV diagnosis was 14 (range 9-16) years. The median follow-up is 12 months (m) (range [R] 6-13). Four children (50%) were asymptomatic and diagnosed by clinical examination/imaging. At diagnosis, visual acuity in the worse eye was 20/100 or better, none had anterior uveitis, while 7 had vitreous cells and vascular leakage (UWFA), 2 neovascularization (UWFA), 3 macular oedema (OCT) and 1 cataract. Laboratory tests (Table 1) were all negative with the exception of Vitamin D (decreased in 3/7). Five of the 8 children were initially treated with oral (n=5) or local/injected corticosteroids (n=4), and anti-VEGF therapy (n=2). Due to persistent inflammation, systemic treatment was started in 7/8 patients. First line treatment was MTX (1 mg/kg [max 20 mg] weekly SQ) that is still ongoing in all (median duration 11 m, R6-12). Because of absent response, IFX (10 mg/kg/dose every 4 weeks) was added in all patients after a median time from diagnosis of 3.2 m (R2.5-3.1) and continued for a median time of 7 m (R3.5-10). However, IFX was ineffective in all patients, and 5/7switched to tocilizumab (10 mg/kg/every 2 weeks IV) after a median time from diagnosis of 9 m (R1-12) with a median duration of 3 m (R0-4). Outcomes at the last available follow-up are reported in Table 1.
Conclusion: We report on the largest series of children with ADNIV treated with systemic immunosuppression. Early testing for CAPN5 gene in at risk children, and regular scheduled screening for uveitis and vasculitis will lead to prompt intervention. MTX and IFX seem ineffective and tocilizumab might be a promising treatment based on underling mechanism.
To cite this abstract in AMA style:
Maccora I, Sood A, Schulert G, Quilan-Water M, Duell A, Huggins J, Nguyen T, Sapp C, Sharma S, Sunil S, Angeles-Han S. Outcomes of Children with Uveitis Associated with Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy (ADNIV) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/outcomes-of-children-with-uveitis-associated-with-autosomal-dominant-neovascular-inflammatory-vitreoretinopathy-adniv/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-of-children-with-uveitis-associated-with-autosomal-dominant-neovascular-inflammatory-vitreoretinopathy-adniv/