Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The 2012 American College of Rheumatology (ACR) gout guidelines recommends allopurinol or febuxostat as first-line urate-lowering therapy (ULT). Purpose of this study was to compare outcomes in ULT-treated patients who were initiated and maintained on either allopurinol or febuxostat. In addition to outcomes of those switched to febuxostat after failing to reach target serum urate (sUA) ≤ 6mg/dL on allopurinol.
Methods: Centricity Electronic Medical Record database of ambulatory care by GE was used. Patients ≥18 years of age, with newly diagnosed with gout (ICD-9, 274.xx) on or after 2005, and initiated on allopurinol or febuxostat were included. Study index date was defined as date of first initiated ULT in patients with at least 6 months of data pre- and post study index. Patients were followed to date of last record. Primary study endpoint was reaching target sUA <6mg/dL within 6 months from index date. Descriptive statistics and bivariate analyses were performed on proportions of patients who reached target sUA among groups of allopurinol-throughout, febuxostat-throughout, or allopurinol-switched-to-febuxostat.
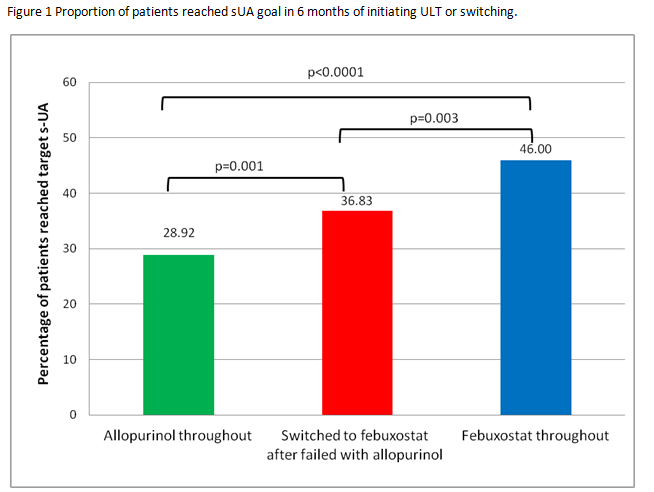
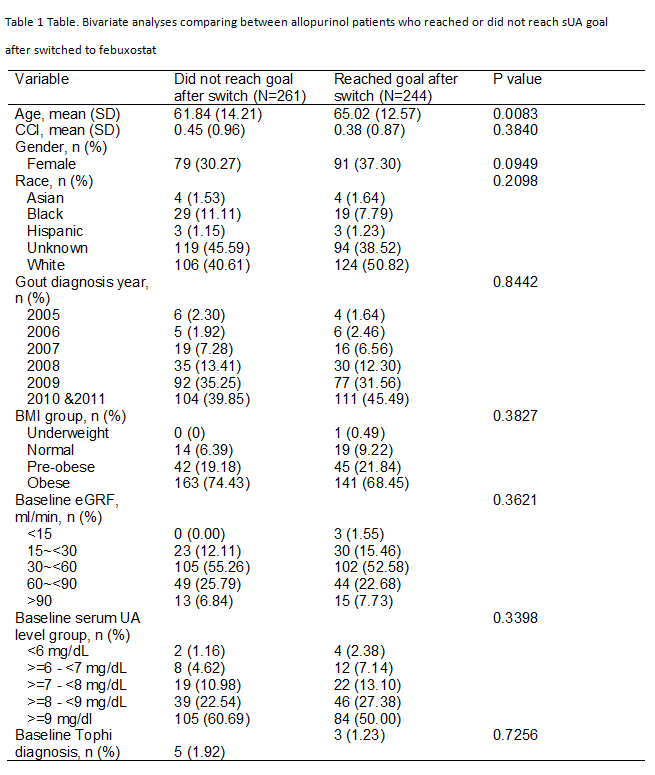
Results: Study cohort consisted of 16,366 patients who used allopurinol throughout, 884 patients used febuxostat throughout, 505 patients switched to febuxostat after failing targeted sUA with allopurinol. Within 6 months after switching to febuxostat, 36.8% of 505 patients who failed to reach target sUA on allopurinol achieved the goal sUA <6mg/dL. The proportion of allopurinol-switched-to-febuxostat who reached target sUA level was significantly higher than that among the allopurinol-throughout patients (36.8 vs. 28.9%, p=0.001), however, significantly lower than that among the febuxostat-throughout patients (36.8% vs. 46.0%, p=0.003, Figure). The 6-month average sUA after switching was 5.4 mg/dL, representing 39% reduction from the mean value of 8.6 mg/dL at time of switch. At the end of available records, 244/505 (48.3%) reached target. For these patients who reached target sUA, the mean (SD) days to switch from allopurinol was 197 (165 days), and the mean days of febuxostat treatment after the switch was 111 (137 days). The only significant difference between those who reached or did not reach sUA target was that the former group was significantly older (Table).
Conclusion: Proportion of patients reaching target sUA levels varied significantly in patients utilizing ULT. Those who switched from allopurinol to febuxostat did significantly better than those who stayed on allopurinol in reaching target sUA but worse than those who started on febuxostat from the beginning.
Disclosure:
H. Hatoum,
Hind Hatoum,
5;
D. Khanna,
Dinesh Khanna,
5,
Dinesh Khanna,
2;
A. Shiozawa,
Takeda Pharmaceuticals International,
3;
S. J. Lin,
Swu-Jane Lin,
5;
K. Akhras,
Kasem Akhras,
3;
P. Khanna,
Puja Khanna,
5,
Puja Khanna,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcome-of-gout-patients-placed-on-febuxostat-after-failing-to-reach-serum-urate-target-with-allopurinol/