Session Information
Date: Tuesday, November 10, 2015
Title: Rheumatoid Arthritis-Small Molecules, Biologics and Gene Therapy VI: Strategies
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose:
Methotrexate (MTX) is the
cornerstone of rheumatoid arthritis (RA) therapy1 but absorption saturation
limitations compromise oral MTX bioavailability (BA). Subcutaneous (SC) MTX has
a dose-proportional, linear absorption profile compared to oral MTX, which plateaus
at doses >15 mg.2 Differences in the relative BA of oral vs SC
MTX demonstrate the need for guidance on successful dose-conversion strategies.
Methods:
In a phase 2, 12-week, open-label,
crossover study, 49 adults with RA already receiving MTX for ≥3 months
were given 10, 15, 20, or 25 mg MTX based on their current MTX dose and disease
control, and then randomized 1:1:1 to oral MTX, SC MTX (abdomen; MTXAIab),
or SC MTX (thigh; MTXAIth). Blood samples for pharmacokinetic (PK)
analysis were collected pre-dose and at 13 time points 0.25 to 12 h post-dose;
samples were analyzed by liquid chromatography–mass spectrometry. Mixed model
analysis derived area under the curve (AUC), maximum observed concentration (Cmax),
and time to maximum concentration (Tmax) PK parameters. Dose-normalized
parameter ratios were calculated.
Results:
Mean age was 61 y, mean body mass
index was 30.7 kg/m2, and mean disease duration was 13 y; 63% of patients
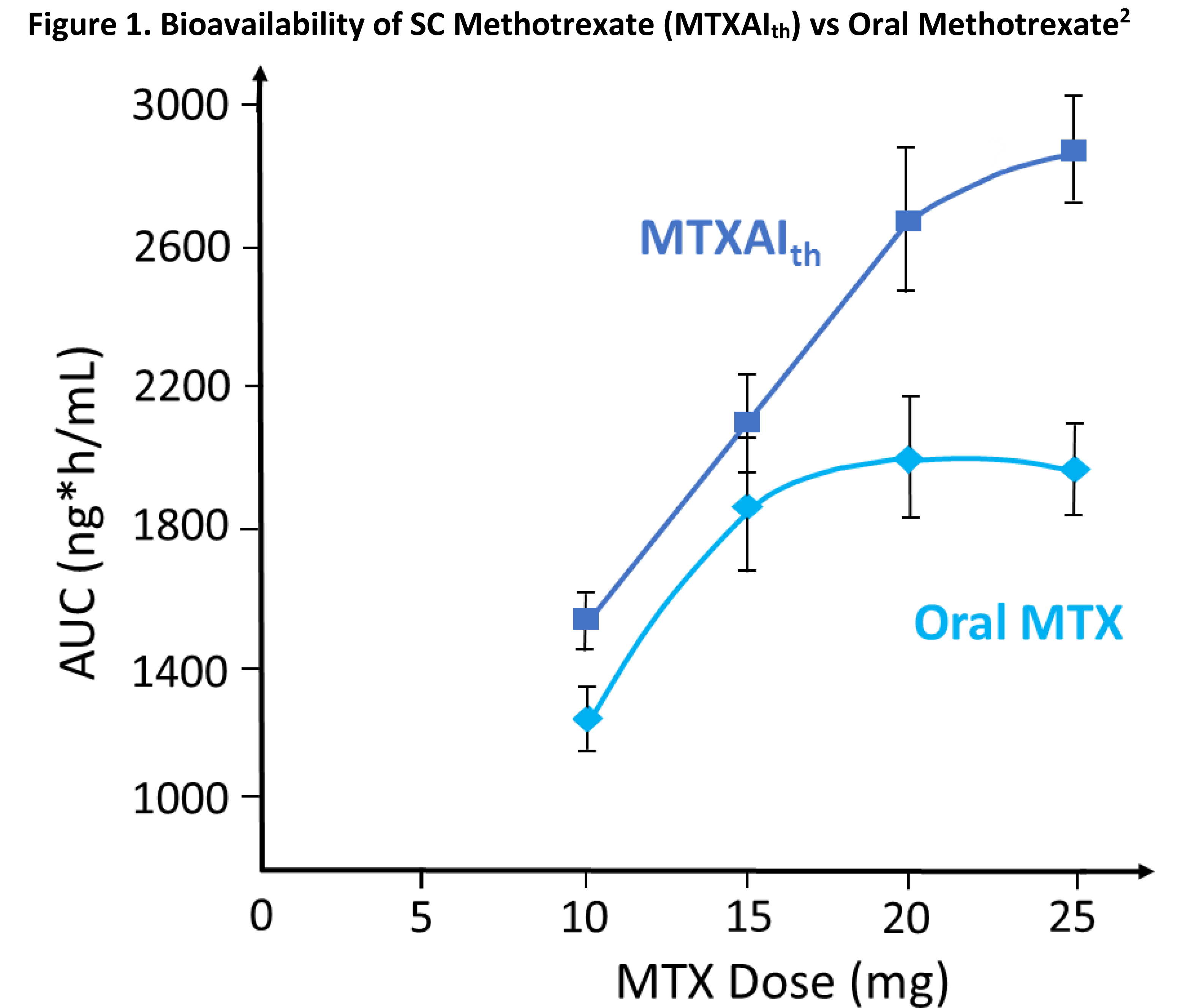
were female. PK analysis of MTXAIth vs oral MTX showed that BA of MTXAIth
was consistently greater at all dose levels (Figure 1). MTXAIth and MTXAIab
PK measures were similar. Although oral MTX plateaued at 15 mg, MTXAI had no
plateau, resulting in higher exposure than comparable oral doses. Relative BA
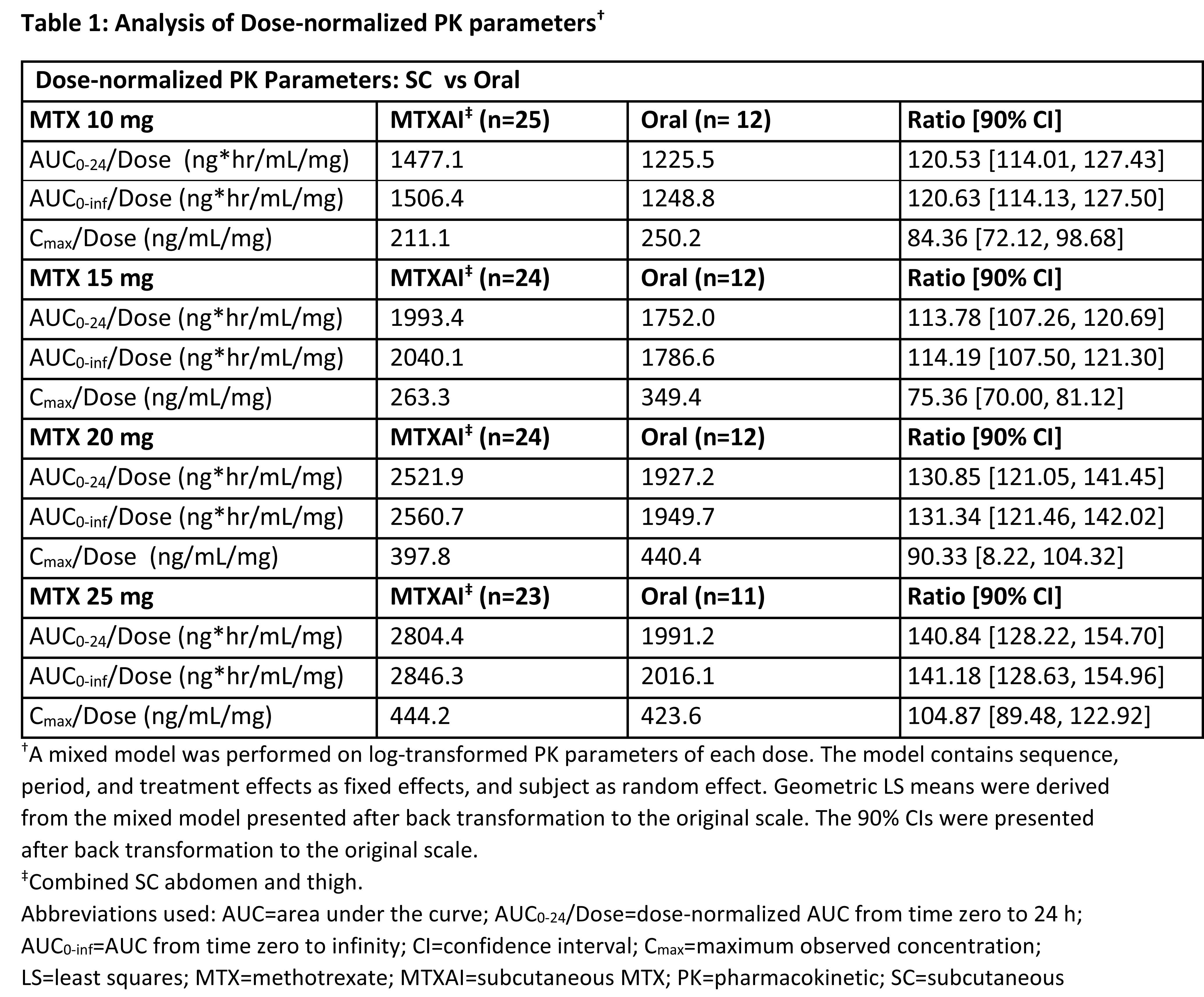
(AUC of MTXAIth vs AUC of oral MTX) at 10, 15, 20 and 25 mg were
121%, 114%, 131%, and 141% respectively (Table 1). Ratios of the
dose-normalized AUC0-24 [90% CI] and Cmax [90% CI] of MTXAI vs oral MTX were
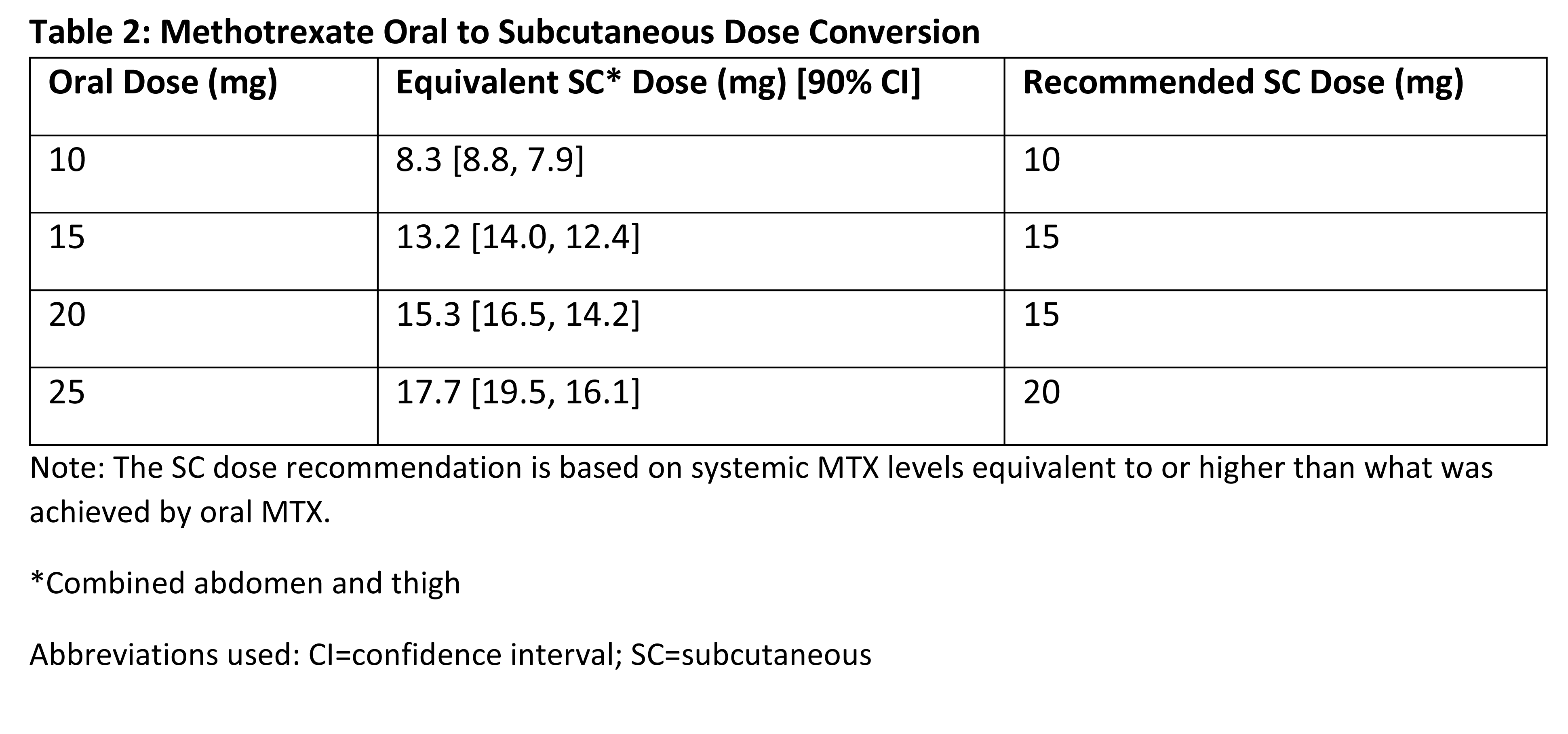
127.61 [122.30, 133.15] and 94.88 [87.95, 102.37]. Equivalence between oral and
SC MTX doses was determined based on BA (Table 2). SC MTX was safe and
well-tolerated in subjects with RA.
Conclusion:
A dose conversion method was
established based on the BA of MTX from oral and SC administration. SC
administration provided higher exposure of MTX than the same dose given orally.
1.
Singh JA, et al. Arthritis Care Res. 2012; 64:625–39
2.
Schiff MH, et al. Ann Rheum Dis. 2014; 73:1549–1551
To cite this abstract in AMA style:
Schiff M, Sadowski P. Oral to Subcutaneous Methotrexate Dose-Conversion Strategies in the Treatment of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/oral-to-subcutaneous-methotrexate-dose-conversion-strategies-in-the-treatment-of-rheumatoid-arthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/oral-to-subcutaneous-methotrexate-dose-conversion-strategies-in-the-treatment-of-rheumatoid-arthritis/