Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: EGPA is a severe, rare, relapsing/remitting inflammatory disease, in which chronic or high oral corticosteroid (OCS) doses lead to adverse effects, adding to disease burden. The efficacy and safety of mepolizumab, an anti-interleukin-5 biologic, was studied in EGPA in a Phase III double-blind placebo-controlled trial (MIRRA). There are currently limited data regarding OCS use following long-term mepolizumab treatment ( >1 year) in patients with EGPA. The Long-term Access Programme (LAP/MEA116841/NCT03298061) provided mepolizumab treatment to patients following completion of MIRRA until approval of mepolizumab. The LAP assessed safety and OCS use following real-world long-term mepolizumab treatment.
Methods: LAP, a multicenter, open-label extension study, enrolled patients with EGPA from MIRRA (49 mepolizumab, 51 placebo) who required prednisolone (or equivalent) ≥5 mg/day within 6 months of MIRRA completion. In LAP, all patients received mepolizumab 300 mg subcutaneously every 4 weeks plus standard-of-care until mepolizumab was discontinued (any reason) or became commercially licensed for EGPA in the relevant country (“completion”). Adverse events and OCS use were recorded. Post hoc OCS endpoints, assessed by 3-month period, included OCS median dose, proportion of patients receiving OCS, and proportion achieving ≥50% reduction in OCS dose relative to MIRRA baseline. Results are summarized descriptively.
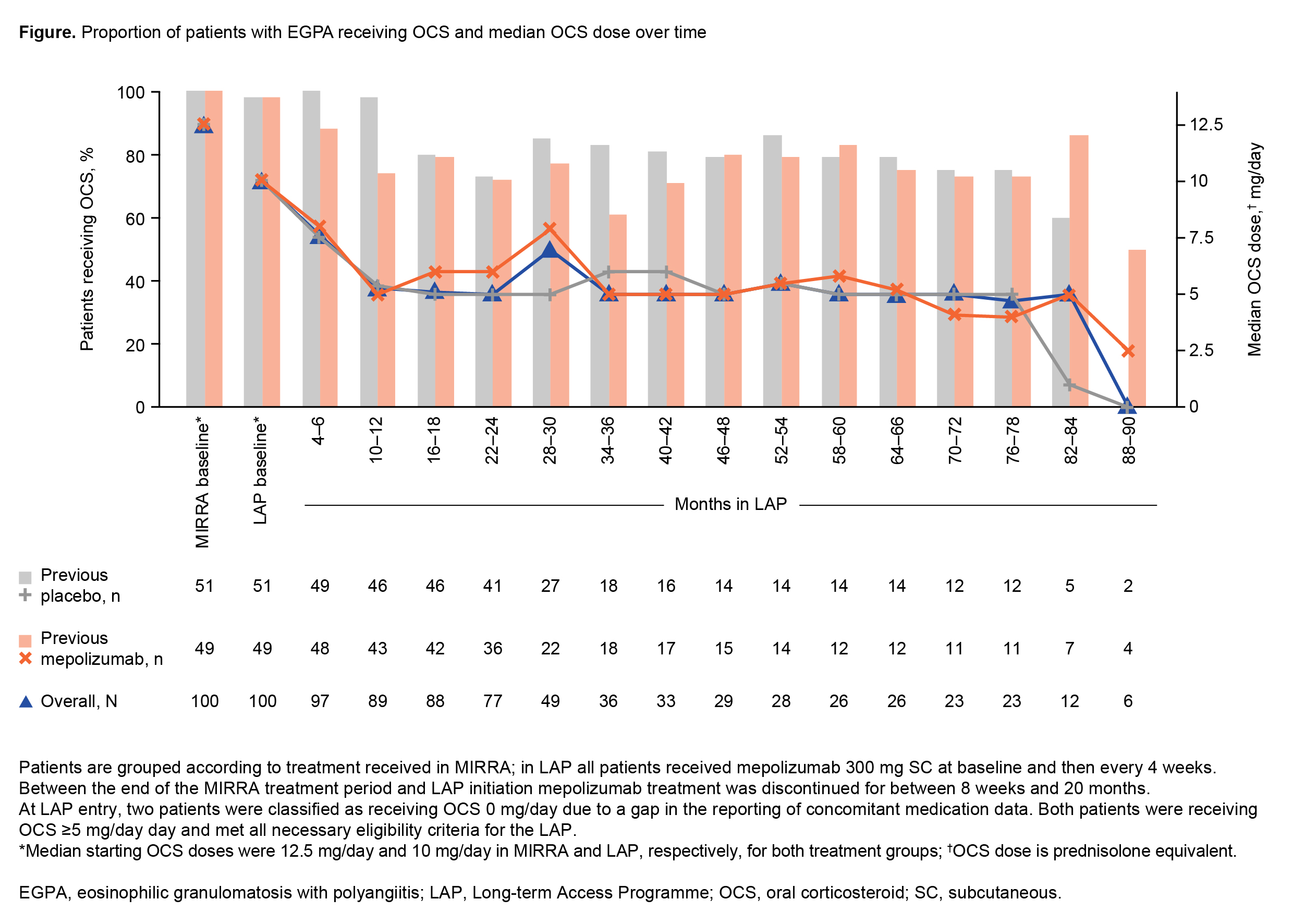
Results: Of 100 patients in LAP, 71% completed treatment and 29% discontinued treatment. Mean (SD) exposure during LAP was 38.5 (27.0) months (89 months maximum [+12 months for MIRRA mepolizumab-treated patients]). The percentage of patients receiving OCS decreased within the first 18 months of LAP, and occurred earlier in patients who had previously received mepolizumab than placebo in MIRRA (Figure). Median (IQR) OCS dose decreased steadily from 10 (7.8, 15.0) mg/day at LAP entry to 5.1 (1.8, 9.1) mg/day, by Months 16–18, and remained low thereafter (Figure). At LAP entry, 75% of participants were taking OCS >7.5 mg/day; this reduced to 51% at Months 4–6, and 32% at Months 16–18. Overall, a ≥50% reduction in OCS compared with MIRRA baseline was achieved by 66% of participants at 16–18 months. Long-term mepolizumab was generally well tolerated, and no new safety signals were identified.
Conclusion: Long-term mepolizumab resulted in a reduction in OCS use, maintained for up to 7.4 years of treatment (+ 1 year prior in those previously randomized to mepolizumab). While OCS treatment was still required for some patients, the overall dosage was lowered in the majority.
Funding: GSK ID: MEA116841/NCT03298061
Abstract previously presented at American Thoracic Society (ATS) International Conference 2024 Annual Scientific Meeting. Am J Respir Crit Care Med. 2024;209:A1382.
To cite this abstract in AMA style:
Khoury P, Silver J, Wolff G, Price R, Verghis R, Igboekwe E, Wechsler M. Oral Corticosteroid-Sparing Effects of Mepolizumab in Eosinophilic Granulomatosis with Polyangiitis (EGPA): Results up to 7.4 Years from the Long-Term Access Programme [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/oral-corticosteroid-sparing-effects-of-mepolizumab-in-eosinophilic-granulomatosis-with-polyangiitis-egpa-results-up-to-7-4-years-from-the-long-term-access-programme/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/oral-corticosteroid-sparing-effects-of-mepolizumab-in-eosinophilic-granulomatosis-with-polyangiitis-egpa-results-up-to-7-4-years-from-the-long-term-access-programme/