Session Information
Date: Tuesday, November 12, 2019
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III: Giant Cell Arteritis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tocilizumab (TCZ) is the only biological agent approved in Giant Cell Arteritis (GCA). There is general agreement on the initial and the standard maintenance dose of TCZ. However, information on duration and optimization of TCZ in GCA is scarce.
Our aim was to assess efficacy and safety of TCZ therapy optimization in an unselected wide series of GCA in clinical practice.
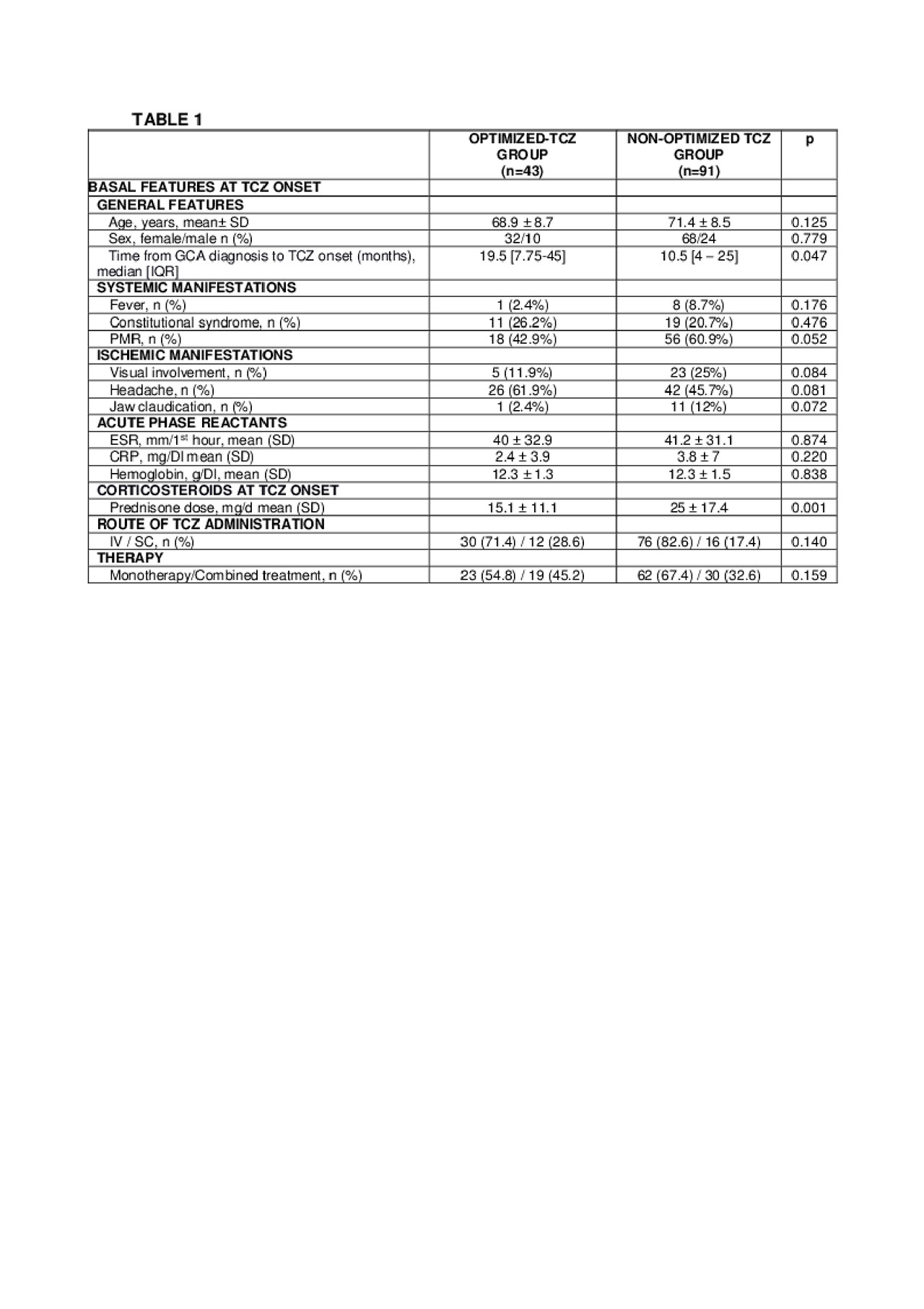
Methods: Multicenter study on 134 patients with GCA who received TCZ therapy due to inefficacy or adverse events of previous therapy. Once complete remission was reached and based on a shared decision between the patient and the physician TCZ was optimized in some cases. Complete remission was defined as normalization of clinical an analytical (CRP and ESR) findings. Optimization was done by decreasing the dose and/or prolonging the TCZ dosing interval progressively. We performed a comparison between optimized and non-optimized patients.
Results: We evaluated 134 GCA patients treated with TCZ (101 women/33 men); mean age 73.0±8.8 years. TCZ was administered IV to 106 (79.1%) patients and SC to 28 (20.9%). The initial dose was 8 mg/kg/IV/4 weeks or 162 mg/SC/week, respectively. TCZ was optimized in 42 (31.3%) patients. No demographic, clinical manifestations or laboratory data differences had been found at TCZ onset between both groups (TABLE1). After a median [25-75th] follow up of 12 [6-15.5] months, and in a complete remission for 6 [3-12] months; the first TCZ optimization was performed in the optimized group. The median prednisone dose at first TCZ optimization was 2.5 [0-5] mg/day. TCZ IV was optimized from 8 to 4 mg/kg/4 weeks in 11 of 106 (10.4%) and from 162 mg/SC/week to 162 mg/SC/2 weeks in 9 of 28 (32.1%) cases. In TABLE 2 data of the optimized doses. Five (11.9%) of the 42 optimized cases relapsed. In 4 of these cases, the relapses were treated increasing TCZ up to the pre-optimization dose, and in 1 case the route of administration of TCZ was change (4 mg/kg/4week to 162 mg/SC/week). In 8 of 42 optimized patients (19%), it was possible to withdraw TCZ after complete remission for 30 [16.25-45.75] months. Regarding adverse events, severe infections were less frequent in the optimized group (14.3% vs 11%) with non-statistical significance.
Conclusion: Once remission is reached in GCA patients under TCZ treatment, optimization of TCZ may be performed. Based on our experience it could be performed by reducing the dose with IV TCZ or by prolonging dosing interval with SC TCZ. It seems to be effective and safe.
To cite this abstract in AMA style:
Calderón-Goercke M, Loricera J, PRIETO- PENA D, Castañeda S, Aldasoro Caceres V, Villa I, Humbría A, Moriano C, Romero-Yuste S, Narváez J, Gómez-Arango C, Perez Pampín E, Melero R, Becerra-Fernández E, Revenga M, Álvarez-Rivas N, Galisteo C, Sivera F, Olivé-Marqués A, Álvarez del buergo M, Marena-Rojas L, Fernández-López C, Navarro F, Raya E, Galindez-Agirregoikoa E, Arca B, Solans-Laqué R, Conesa A, Hidalgo C, Vazquez C, Román-Ivorra J, Lluch P, Manrique S, Vela P, de Miguel E, Torres-Martín C, Nieto J, Ordas-Calvo C, salgado-Pérez E, Luna-Gómez C, Toyos-Sáenz De Miera F, Fernández-Llanio N, García A, Larena C, Varela-García M, Aurrecoechea E, Ortiz-Sanjuán F, Hernández J, González-Gay M, Blanco R. Optimization of Tocilizumab Therapy in Giant Cell Arteritis: A Multicenter Real Life Study of 134 Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/optimization-of-tocilizumab-therapy-in-giant-cell-arteritis-a-multicenter-real-life-study-of-134-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/optimization-of-tocilizumab-therapy-in-giant-cell-arteritis-a-multicenter-real-life-study-of-134-patients/