Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Colchicine decreases cytokine production by inhibiting NLRP3 inflammasome activation and is considered effective in treating COVID-19. Although two randomized controlled trials failed to demonstrate a therapeutic effect of colchicine, in one of these trials, 94% of the patients had already received glucocorticoids before randomization, making the anti-inflammatory effect of colchicine impossible to assess.
Methods: During the spread of the delta variant of SARS-CoV-2, patients with mild to moderate COVID-19 symptoms were admitted to an ad hoc ward in our hospital that was managed mainly by rheumatologists. The present study compared the patients admitted to this ward between August 4 and August 23, 2021 who did not receive colchicine with those who were admitted to the same ward between August 24 and September 30, 2021 who did. Patients who received oxygen from the day of admission were excluded. As per protocol, dexamethasone and anticoagulants were started when oxygen saturation fell to 93% or less at rest. No antiviral agent (e.g., remdesivir) was given to oxygen-free patients. From August 4, 2021, REGN-COV2 (casirivimab and imdevimab) was added to the protocol. From August 24, 2021, colchicine was also added. If oxygen was not initiated on the day of admission, colchicine was started at 0.5 mg twice daily, then adjusted to 0.5 mg/day – 1.5 mg/day as appropriate. Colchicine administration was stopped before discharge. When oxygen therapy was begun, colchicine was switched to dexamethasone.
To adjust for intergroup differences, inverse probability treatment weighting (IPTW) was conducted. The present, retrospective, observational study was approved by the institutional ethics review board of Tokyo Metropolitan Tama Medical Center (#3-145).
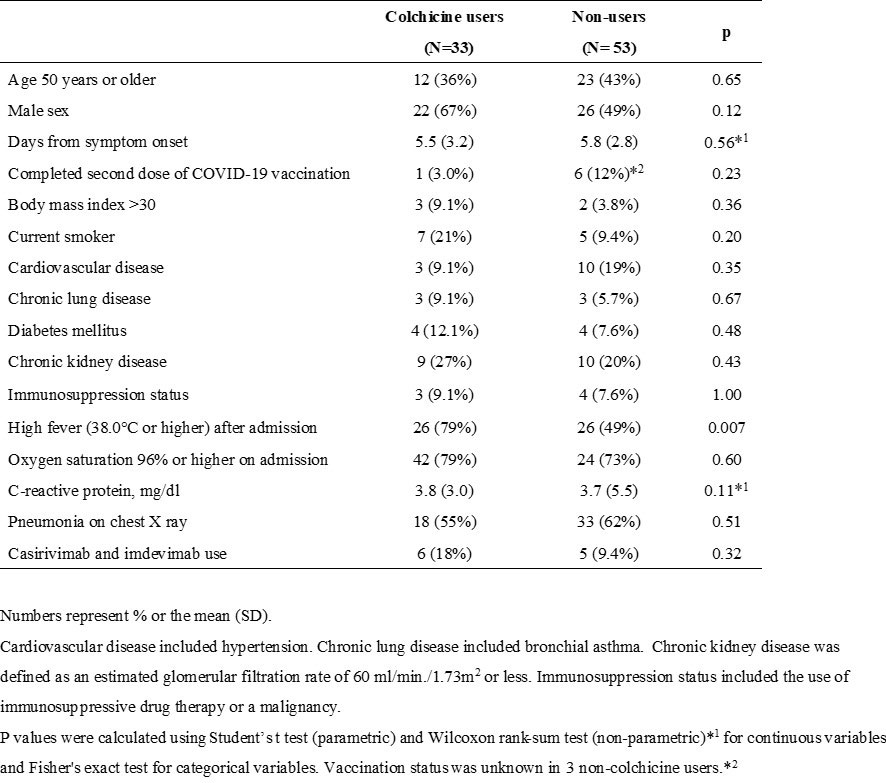
Results: Table 1 shows the baseline characteristics of both patient groups. A high fever (38.0 degrees or higher) after admission was recorded on Day 1 and/or Day 2 in all the patients and was associated with colchicine use (odds ratio [OR]: 3.86; 95% confidence interval [CI]: 1.48-11.0.; P=0.005). All variables listed in Table 1 were used in IPTW adjustment.
The colchicine dosage was decreased in 12% (4/33), and increased in 3% (1/33), of the patients. The mean (SD) number of days of colchicine use was 4.2 (2.6). No patient died or required a ventilator. The rate of oxygen administration was 15% (5/33) and 37% (20/53) in patients with and without colchicine, respectively (p=0.029). Among those with a high fever after admission, the rate was 19% (5/26) and 54% (14/26) (p=0.020), respectively.
Colchicine was associated with a significantly lower risk of oxygen administration in an unadjusted analysis (OR: 0.295; 95% CI: 0.098-0.887; P=0.030) and after adjustment using IPTW (OR: 0.298; 95% CI: 0.095-0.934; P=0.038).
Conclusion: The present, observational study suggested that colchicine is most effective in febrile patients with imminent respiratory failure before glucocorticoid administration and formed the basis for an open-label, randomized, parallel group trial called, “Colchicine for preventing respiratory failure in COVID-19 with fever (COLPREF),” which we began during the current SARS-CoV-2 pandemic (jRCTs031210579).
To cite this abstract in AMA style:
Yokogawa N, Iida M, Onishi K, Asadori D, Ueda Y, Kanematsu E, Saegusa K, Sano T, Morishima R, Hayashi K, Hataya H, Nishida K. Optimal Use of Colchicine in COVID-19 Management from Rheumatologists’ Perspective: A Monocentric Observational Study During the SARS-CoV-2 Delta Variant Pandemic [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/optimal-use-of-colchicine-in-covid-19-management-from-rheumatologists-perspective-a-monocentric-observational-study-during-the-sars-cov-2-delta-variant-pandemic/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/optimal-use-of-colchicine-in-covid-19-management-from-rheumatologists-perspective-a-monocentric-observational-study-during-the-sars-cov-2-delta-variant-pandemic/