Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: With the recent introduction of biological DMARDs, the economic burden of rheumatoid arthritis (RA) treatment has increased. Previous studies evaluated the cost-effectiveness of biologics, although those studies were usually conducted based on controlled clinical trials, which do not precisely reflect daily practice. From an economic point of view, it is desirable to treat patients with biologics when the cost-effectiveness is maximized, especially in daily practice. We conducted a pharmacoeconomic study to investigate the optimal timing of biologics administration to RA patients using the RA cohort database of the Institute of Rheumatology, Rheumatoid Arthritis (IORRA).
Methods: We conducted a state-transition model-based probabilistic simulation from the societal perspective. Model parameters were determined using clinical data for 421 patients from the IORRA who had failed at least one DMARD and had started either one of four biologics (adalimumab, etanercept, infliximab, and tocilizumab) or methotrexate (MTX) between April 2008 and April 2011. Patients who had not used biologics were matched using propensity scores. A hypothetical population of 10,000 patients treated with sequences of three biologics after MTX and then best supportive care (BSC) were compared to those starting MTX after BSC. The incremental cost-effective ratios (ICERs) of the biologics groups against the MTX group were estimated using base-case analysis and probabilistic sensitivity analysis. Scenario sensitivity analyses were also done for different backgrounds of the initial population classified by age, disease duration, and disability based on scores of the Japanese version of HAQ (J-HAQ). Because tocilizumab differed from TNF-blockers in mechanism and had a large decrease in price in Japan in 2012, sequences of three of four biologics and three except for tocilizumab were also considered for biologics groups.
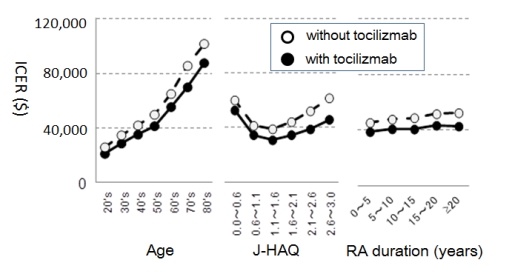
Results: The ICERs of treatment sequences with biologics and with or without tocilizumab were $38,180 and $48,855, respectively. By probabilistic sensitivity analysis, these sequences had respective probabilities of 89.5% and 80.9% of falling below an assumed threshold of $54,000 as the societal willingness-to-pay in Japan. Scenario sensitivity analyses showed that the most influential factors on the ICER were age and J-HAQ scores. Starting biologics at a younger age had better cost-effectiveness, whereas disease duration had no effect. J-HAQ scores followed “J-curves” and the ICER was best for patients with J-HAQ scores of 1.1 to 1.6 (Figure).
Conclusion: Our results demonstrate that biological DMARDs are cost-effective for RA patients based on data from an observational cohort representing daily practice in Japan. From pharmacoeconomic perspectives, the best population for biologics use is younger RA patients with J-HAQ baseline scores between 1.1 and 1.6 and using tocilizumab as the biological DMARD.
Disclosure:
E. Tanaka,
None;
E. Inoue,
None;
Y. Shimizu,
None;
A. Kobayashi,
None;
N. Sugimoto,
None;
D. Hoshi,
None;
K. Shidara,
None;
E. Sato,
None;
Y. Seto,
None;
A. Nakajima,
None;
S. Momohara,
None;
A. Taniguchi,
None;
H. Yamanaka,
Abbott, AbbVie, Asahikasei , Astellas, AstraZeneca, Bristol-Myers Squib, Chugai, Daiichi Sankyo, Eisai, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, MSD, Nippon Kayaku, Pfizer, Santen, Taishotoyama, Takeda, Teijin,
2,
Abbott, AbbVie, Astellas, AstraZeneca, Bristol-Myers Squib, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Nippon Kayaku, Pfizer, Takeda, Teijin,
5,
Abbott, AbbVie, Astellas, Bristol-Myers Squib, Chugai, Eisai, Mitsubishi Tanabe, Pfizer, Takeda, Teijin,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/optimal-rheumatoid-arthritis-patient-selection-for-biological-treatment-from-pharmacoeconomic-perspectives-based-on-the-institute-of-rheumatology-rheumatoid-arthritis-iorra-cohort/