Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Pregnancies in women with systemic lupus erythematosus (SLE) often result in preterm birth. Active disease during pregnancy significantly increases the risks for these poor outcomes. Hydroxychloroquine (HCQ) reduces disease activity and flares in SLE, however, low serum levels may lead to treatment failure. Therefore, our objective is to evaluate HCQ levels during pregnancy and determine target serum levels to reduce maternal disease activity and preterm birth.
Methods: We performed a single-center observational study of women with SLE who were taking HCQ during pregnancy between 2013 and 2019. Serum samples were analyzed using validated high-performance liquid chromatography/mass spectrometry. Based on prior publications, we analyzed average HCQ exposure both continuously and categorically: ≤ 100 ng/ml (low), 101-500 ng/mL (moderate), and > 500 ng/ml (high). The primary maternal outcome was disease activity, measured using the Physician Global Assessment (PGA) throughout pregnancy. The primary neonatal outcome was gestational age at birth. We excluded pregnancies in which the woman had lupus nephritis within the prior 3 years due to potential confounding between drug levels and preterm birth. We analyzed categorical outcomes using Fisher’s exact test and continuous outcomes using linear regression models, Wilcoxon signed-rank test, Kruskal-Wallis test, t test, and ANOVA.
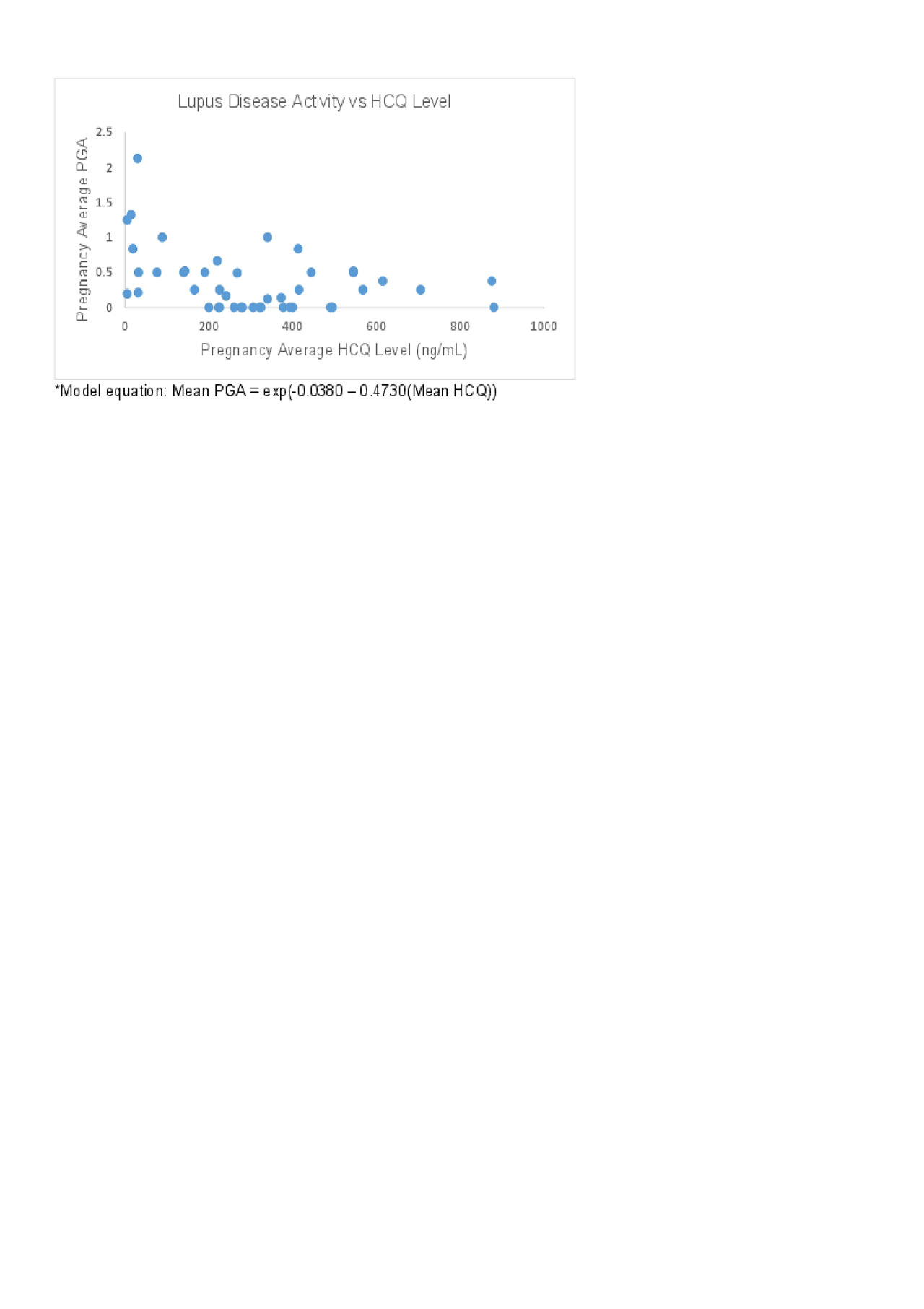
Results: We analyzed 172 samples from 52 pregnancies in 46 women (Table 1). HCQ concentration varied widely among individuals at each trimester. In addition, there was a non-statistically significant decline in HCQ concentrations throughout pregnancy, with a nadir in the 3rd trimester. Of live births (n=38), preterm birth was significantly more common in mothers whose average HCQ level was low (62.5%), compared to those with moderate levels (8.3%), and high levels (33.3%, p=0.005), Table 2. In addition, disease activity in these mothers was significantly higher in those with low HCQ levels (median [IQR]) PGA 0.67 [0.36-1.16], compared to those with moderate levels (0.06 [0-0.37]), and high levels (0.38 [0.25-0.5], p=0.006). The association between PGA and HCQ levels as a continuous variable was best characterized using a generalized linear model with a log function (figure 1, p=0.002).
Conclusion: Our data suggests that the optimal serum HCQ level in this cohort appears to be in the moderate range (101-500 ng/ml), with this group having the lowest maternal disease activity and the fewest preterm births. Women with the lowest HCQ levels, in the range concerning for medication non-adherence, had the highest lupus activity and rates of preterm birth. However, the relationship between HCQ drug levels and neonatal outcomes are complex and not linear, and some women with high exposure (levels >500 ng/mL) continue to experience SLE activity and preterm birth. Due to low sample sizes within subgroups, ongoing data collection and analyses will clarify the role of monitoring HCQ levels in lupus pregnancies, characterize the relationship between drug levels and medication adherence, and determine the need for HCQ dose adjustment through PK/PD modeling.
To cite this abstract in AMA style:
Balevic S, Cohen-wolkowiez M, Eudy A, Becker M, Schanberg L, Clowse M. Optimal Hydroxychloroquine Drug Levels in Pregnant Women with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/optimal-hydroxychloroquine-drug-levels-in-pregnant-women-with-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/optimal-hydroxychloroquine-drug-levels-in-pregnant-women-with-systemic-lupus-erythematosus/