Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Novel composite responder definitions for fibromyalgia (FM) clinical trials have been proposed by the Outcome Measures in Rheumatology (OMERACT) FM subcommittee. These definitions which include key symptom and functional domains relevant to FM patients, were validated using outcome data from 12 previous registration trials of 4 other medications (Arnold L et al, Arth Rheum 64:885, 2012.) TNX-102 SL* is a proprietary sublingual formulation of cyclobenzaprine hydrochloride (2.8 mg) being studied for conditions with sleep disruption, including fibromyalgia and post-traumatic stress disorder (PTSD). “BESTFIT” was a 12-week Phase 2, randomized, double-blind, placebo-controlled trial conducted at 17 US sites, designed to evaluate this medication as a potential treatment for FM. A total of 205 participants were randomized (TNX=103; placebo=102).
Methods: The two recommended OMERACT responder definitions were evaluated: the FM30 Short, which defines a responder as ≥30% reduction in pain, ≥10% improvement in physical function plus a ≥30% improvement in either sleep or fatigue, and the FM30 Long, which defines a responder as ≥30% reduction in pain, ≥10% improvement in physical function plus ≥30% improvement in any two of the following measures: sleep, fatigue, depression, anxiety or cognition. Individual patient level analyses determine if each participant in the study met the response criteria.
Results: The table below compares a standard pain responder analysis (≥30% improvement in pain based on daily diary data), which was the secondary efficacy endpoint in the “BESTFIT” study, to the alternative composite responder definitions proposed by OMERACT. 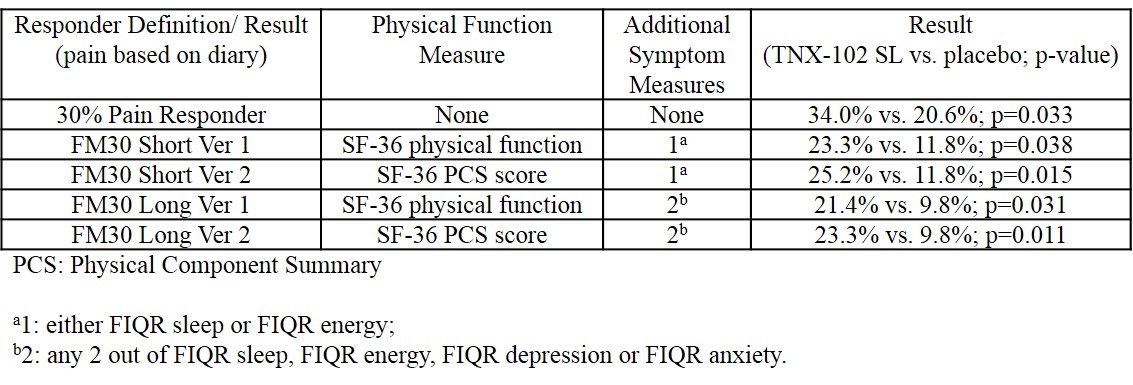
Additional improvements in other domains were noted as well. Systemic adverse events reported were similar to placebo. The most common local adverse event was transient tongue or mouth numbness occurring in 44% of the TNX-102 SL patients and in 2% of placebo patients.
Conclusion: Routine bedtime usage of TNX-102 SL improved multiple symptoms of FM. Comparison of response rates determined by either 30% reductions in pain alone, or by the proposed composite definitions, were all statistically significant. Although the apparent response rate for the composite definitions is necessarily less than that seen with the pain response only analysis, the actual statistical separation between treatment groups is enhanced. This analysis using the composite responder criteria developed by OMERACT suggests that the improvements in FM symptoms in this study of TNX-102 SL may not be limited to an analgesic response, since these composite criteria also require improvements in other somatic and functional symptoms.
* TNX-102 SL is an Investigational New Drug and has not been approved for any indication.
To cite this abstract in AMA style:
Gendreau RM, Arnold L, Clauw DJ, Gendreau J, Daugherty B, Lederman S. Novel Composite Responder Endpoints for Fibromyalgia Therapy Assessment [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/novel-composite-responder-endpoints-for-fibromyalgia-therapy-assessment/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-composite-responder-endpoints-for-fibromyalgia-therapy-assessment/
