Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Treatments Poster II: PROs, Safety and Comorbidity
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
No effect of concomitant glucocorticoid therapy on efficacy and safety of tocilizumab monotherapy in rheumatoid arthritis clinical trials
M. Safy1, J.W.G. Jacobs1, M. Edwardes2, M.J.H. de Hair1, X.M. Teitsma1, P.M.J. Welsing1, M.E.A. Borm3, Y. Luder4, J.M. van Laar1, A. Pethö-Schramm4, J.W.J. Bijlsma1
1 Dept. of Rheumatology & Clinical Immunology, University Medical Center Utrecht, Utrecht University, Utrecht, the Netherlands, 2 Everest Clinical Research, Canada, 3 Roche Nederland BV, Woerden, the Netherlands, 4 F Hoffmann-La Roche, Basel, Switzerland
Background/Purpose: Among RA patients in clinical trials, background treatment with glucocorticoids (GCs) is common. The potential impact of GCs on safety of the DMARD tested in these trials has rarely been evaluated. Our aims were to establish whether a stable concomitant GC treatment influenced: 1) efficacy and safety of tocilizumab (TCZ) monotherapy initiated in RA patients in TCZ RCTs and 2) efficacy and safety in the comparator arms of these trials, in which adalimumab (ADA) or methotrexate (MTX) was initiated.
Methods: Data were used from 4 RCTs including AMBITION, ACT-RAY, ADACTA and FUNCTION with TCZ monotherapy arms. Patients had discontinued bDMARDs or were bDMARD naïve and MTX-naïve, MTX-intolerant or MTX inadequate responders. Stable GC dose at baseline was allowed. Differences in change from baseline up to week 24 in CDAI and DAS28 between GC-users and non-GC-users were analysed using ANCOVA. Difference in radiographic progression up to week 104 between GC-users and non-GC-users was analysed. Incidence rates of SAEs were assessed by GC use.
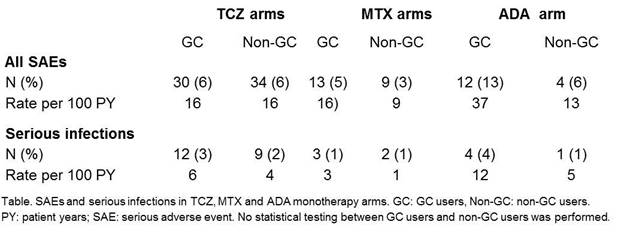
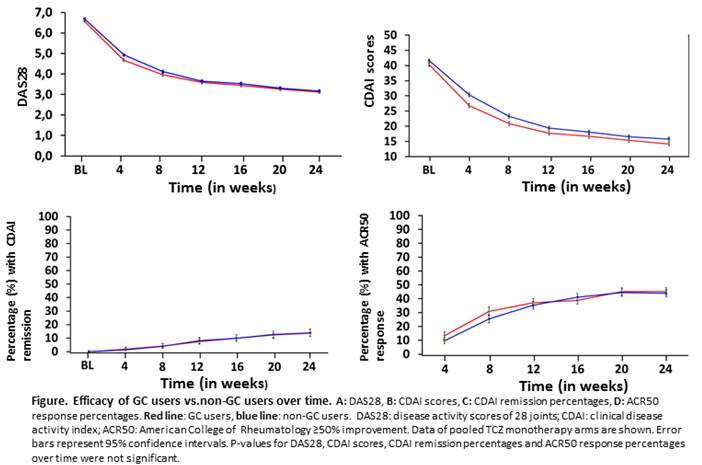
Results: Baseline characteristics were comparable between GC users and non-GC users within RCTs. No significant differences were found in DAS28 change or CDAI change from baseline to 24 weeks, nor in CDAI remission percentages and ACR50 response at 24 weeks, between GC users and non-GC users in TCZ, ADA, or MTX arms (Figure). In the MTX arm a significant difference in radiographic progression was found, in favour of GC use. SAE rates were numerically higher for GC users vs non-GC users in MTX and ADA arms (Table), however this difference was not tested statistically.
Conclusion: In 4 RA TCZ clinical trials, no effect of stable concomitant GC treatment at baseline and continued during the trial was found on efficacy of MTX or TCZ or ADA monotherapy. The SAE rate was numerically higher in GC users compared to non-GC users in the MTX and ADA monotherapy arms.
To cite this abstract in AMA style:
Safy M, Jacobs JWG, Edwardes M, de Hair MJ, Teitsma XM, Welsing PM, Borm ME, Luder Y, van Laar J, Pethö-Schramm A, Bijlsma JWJ. No Effect of Concomitant Glucocorticoid Therapy on Efficacy and Safety of Tocilizumab Monotherapy Found in Rheumatoid Arthritis Clinical Trials [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/no-effect-of-concomitant-glucocorticoid-therapy-on-efficacy-and-safety-of-tocilizumab-monotherapy-found-in-rheumatoid-arthritis-clinical-trials/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/no-effect-of-concomitant-glucocorticoid-therapy-on-efficacy-and-safety-of-tocilizumab-monotherapy-found-in-rheumatoid-arthritis-clinical-trials/