Session Information
Date: Monday, November 11, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster II: Treatments, Outcomes, & Measures
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Due to demographic changes an increasing number of persons reach an age above 70 years. Therefore, the adequate therapy of elderly patients with rheumatoid arthritis (RA) is an increasingly important topic. The aim of this study was to compare treatment continuation of several biologic (b)DMARDs in patients ≤ 65 with elderly patients > 70 years, stratified by onset of disease (young onset (< 65 years) and late onset (≥ 65 years)) and by seropositivity.
Methods: The German register RABBIT is a prospective longitudinally followed cohort of RA patients enrolled with a new start of a DMARD after at least one conventional synthetic (cs)DMARD failure. For the current analysis patients who were enrolled with a bDMARD between 01/2007 and 04/2018 were included. Kaplan Meier methods were applied to analyse treatment continuation.
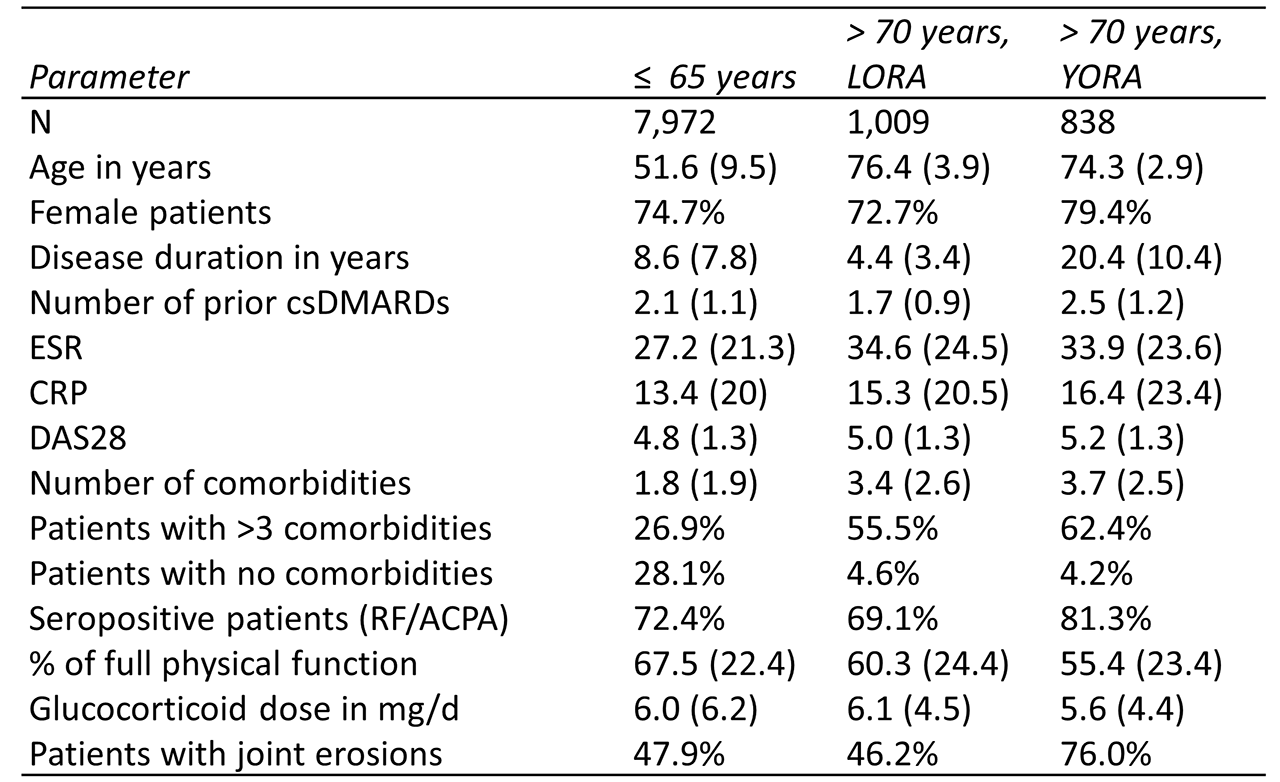
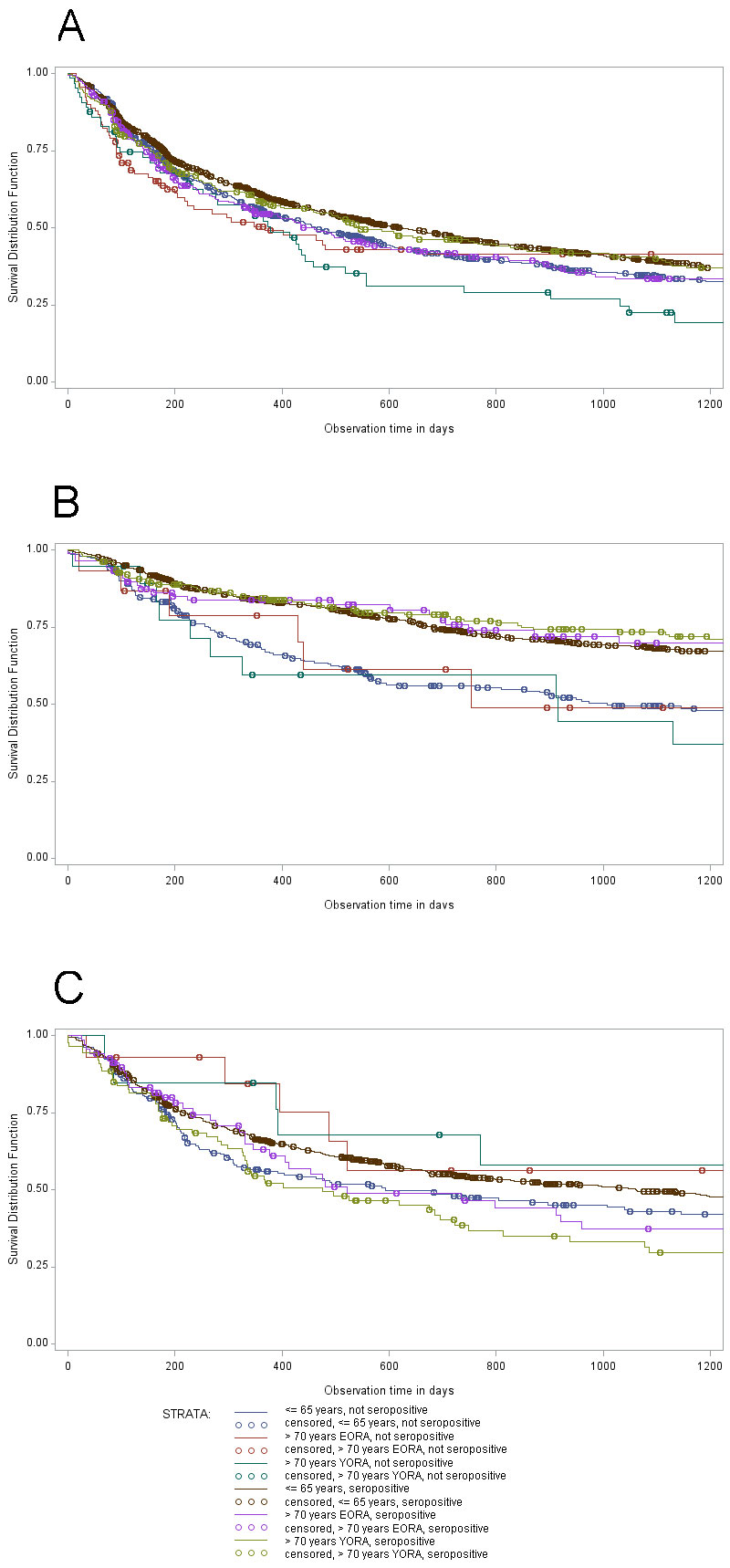
Results: Among the 9,819 RA patients included in the analysis, 7,972 were ≤ 65 years old and 1,847 were older than 70 years (among them 180 patients above 80 years). Among the patients ≤ 65 years, 28% received a csDMARDs and 72% a bDMARD, while among the patients above 70 years, 35% received a csDMARDs and 65% a biological. Elderly patients with a young disease onset (YORA) were more frequently women and more frequently seropositive, on average had a higher number of prior csDMARD treatment failures, a worse physical function and were more likely to have joint erosions than elderly patients with late disease onset (LORA) (Table 1). On all bDMARD treatments investigated, elderly RA patients showed the same treatment continuation as seen in younger patients. While neither the age of the patients nor the age at disease onset changed the continuation of biologicals, patients being seronegative had a significantly lower continuation with rituximab and abatacept treatment, irrespective of age (Figure 1).
Conclusion: These results suggest that bDMARD treatment may be used for elderly patients with the same effectiveness as in younger patients.
Where not otherwise indicated numbers are mean -SD-
To cite this abstract in AMA style:
Strangfeld A, Krüger K, Manger B, Kneitz C, Zink A, Schaefer M. No Difference in Treatment Continuation of Different Biologics in Elderly Patients > 70 Years Compared to Younger Patients ≤ 65 Years [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/no-difference-in-treatment-continuation-of-different-biologics-in-elderly-patients-70-years-compared-to-younger-patients-%e2%89%a4-65-years/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/no-difference-in-treatment-continuation-of-different-biologics-in-elderly-patients-70-years-compared-to-younger-patients-%e2%89%a4-65-years/