Session Information
Date: Sunday, November 12, 2023
Title: (0252–0282) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Autoinflammatory diseases (AID) have been treated safely and effectively with the interleukin-1β inhibitor canakinumab (CAN) in controlled trials and routine clinical practice. The most common adverse event reported were infections. In this study infections and infection rates in patients with cryopyrin-associated periodic syndromes (CAPS), familial Mediterranean fever (FMF), hyper-IgD syndrome/mevalonate kinase deficiency (HIDS/MKD) and tumor necrosis factor receptor-associated periodic syndrome (TRAPS) on CAN therapy were investigated in a real-world setting.
Methods: RELIANCE is a prospective, non-interventional, observational study in Germany enrolling pediatric (age ≥2 years) and adult patients with a clinically confirmed diagnosis of AID who routinely receive CAN. Efficacy and safety parameters are recorded at baseline and assessed at 6-month intervals.
Results: The present interim analysis is based on data from a total of n=232 patients including n=101 (44%) pediatric patients under 18 years diagnosed with autoinflammatory diseases enrolled in the RELIANCE registry. The median duration of CAN treatment before and during study in the pediatric cohort was 4 years (0−15 years).
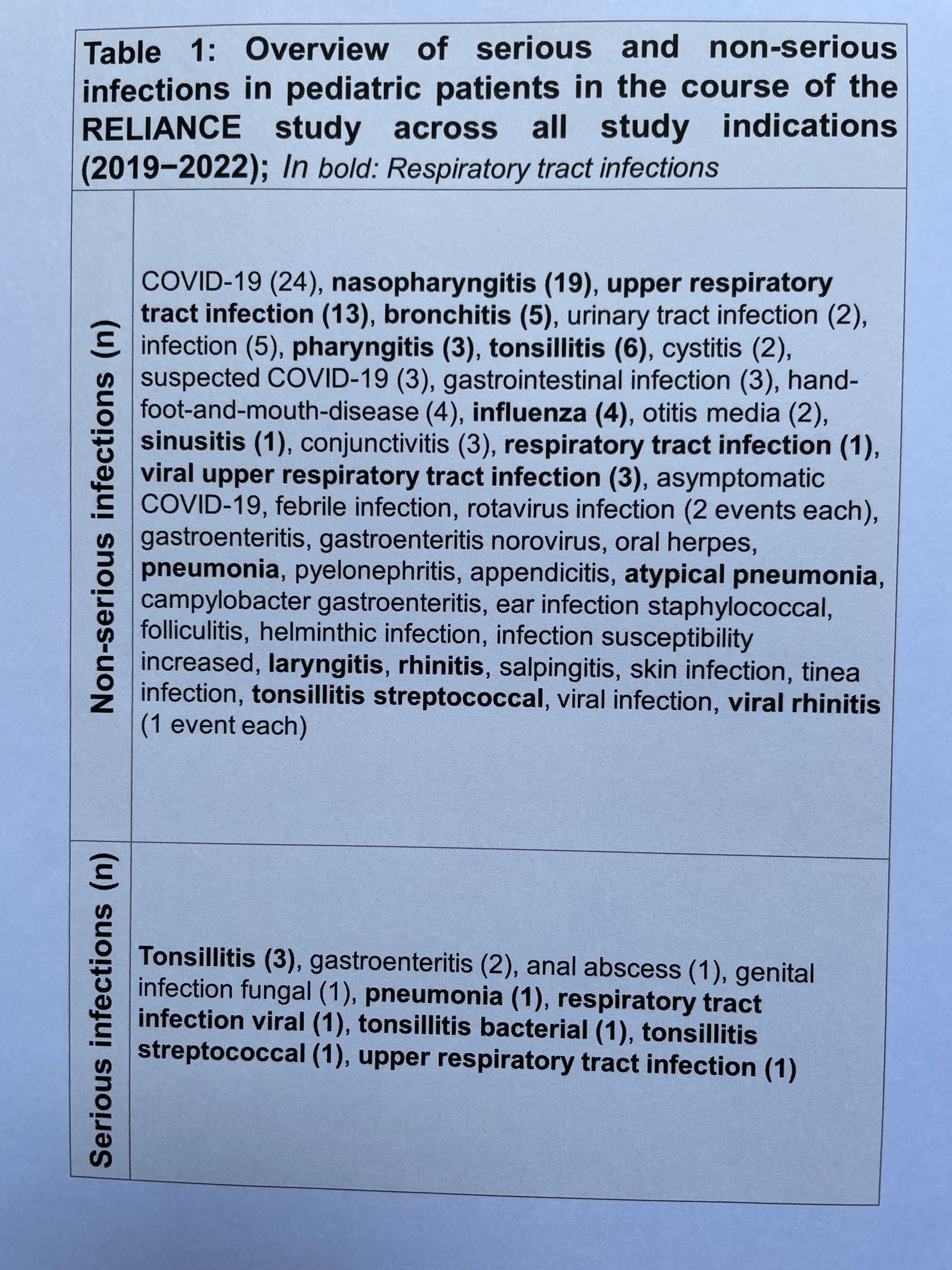
Between 2017 and 2022, 898 adverse events (AE) were recorded in n=164 patients (71%; Table 1). The incidence rate per 100 patient years (IR) was 163.82. Serious adverse events (SAE) were reported for n=35 patients (15%; 98 events, IR 17.88).
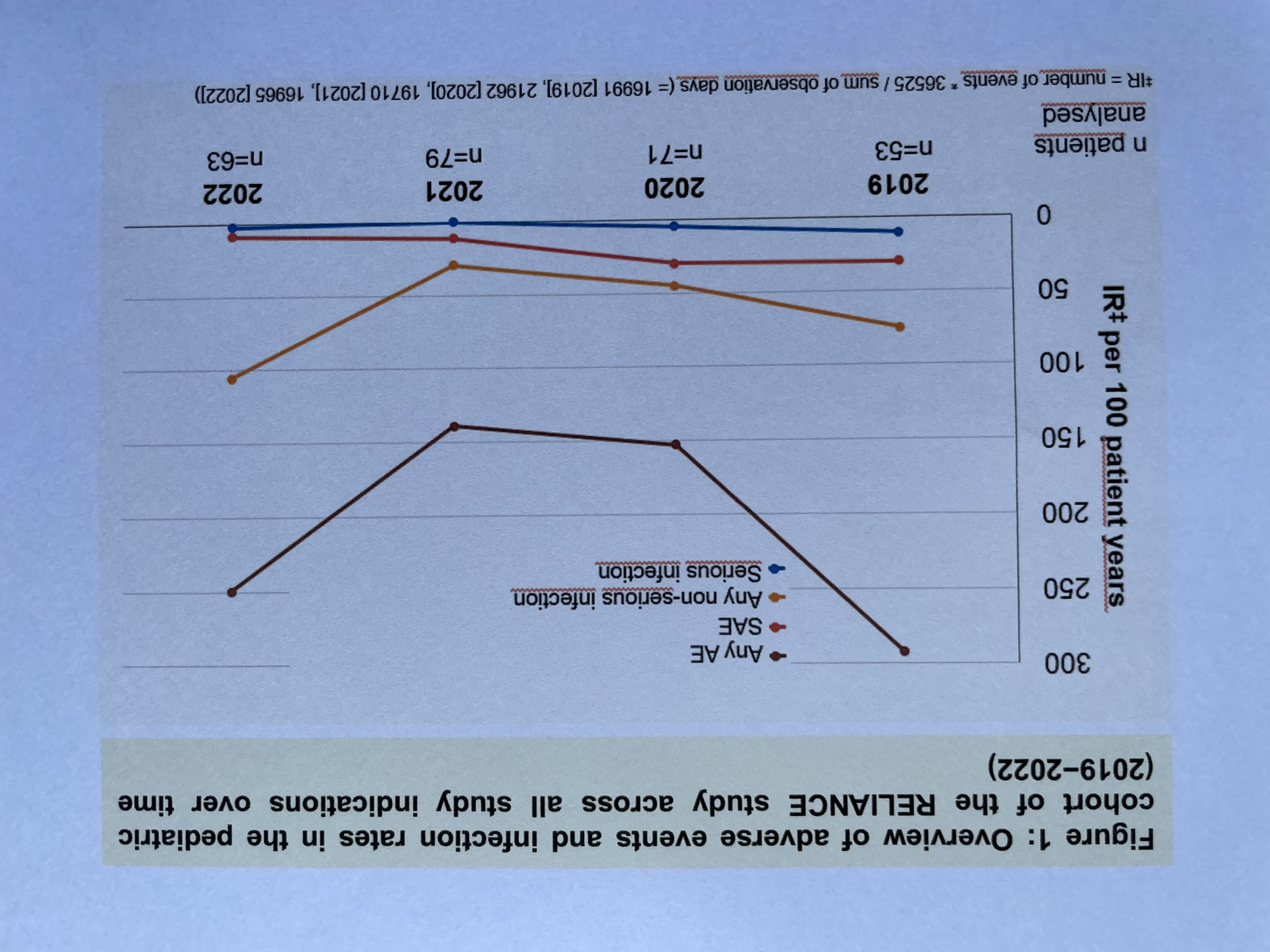
During the study, infections occurred in 54.5% of patients (55 patients, 131 AE, including 11 SAE). To closely monitor the impact of long-term CAN treatment on infection rates in pediatric patients, data from a total of n=53, 71, 83 and 80 pediatric patients enrolled in the study in 2019, 2020, 2021, and 2022 were compared (Fig. 1). The IR of non-serious and serious infections in pediatric patients was 75.24 and 10.75 in 2019, and dropped to 44.90 and 4.99 in 2020, and 29.65 and 0.00 in 2021. The IR decrease might be caused by the periods of social distancing during the coronavirus pandemic in 2020 and 2021. In 2022, the IR of non-serious infections increased to 105.50 while the IR of serious infection stayed flat (IR 2.15). The course of upper respiratory tract infections IR in 2019, 2020, 2021, and 2022 was comparable to the IR of non-serious infections: 10.75, 8.32, 1.85, and 10.76. No cumulation of non-serious and serious infections under Canakinumab long-term treatment could be observed in the pediatric cohort.
Conclusion: Interim data of the RELIANCE study confirm that in the pediatric cohort the risk of infections including upper respiratory tract infections does not accumulate over 4 years under CAN treatment.
To cite this abstract in AMA style:
Kuemmerle-Deschner J, Henes J, Kortus-Goetze B, Oommen P, Pankow A, Kallinich T, Krickau T, Schuetz C, Horneff G, Foeldvari I, Rech J, Weller-Heinemann F, Janda A, Hufnagel M, Meier F, Dressler F, Borte M, Andreica I, Wasiliew P, Fiene M, Windschall D, Krusche M, Kuempfel T, Weber-Arden J, Blank N. No Cumulative Effect of Infection Rates in Children Receiving Long-term Canakinumab Treatment in Autoinflammatory Periodic Fever Syndromes − Data from the RELIANCE Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/no-cumulative-effect-of-infection-rates-in-children-receiving-long-term-canakinumab-treatment-in-autoinflammatory-periodic-fever-syndromes-%e2%88%92-data-from-the-reliance-registry/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/no-cumulative-effect-of-infection-rates-in-children-receiving-long-term-canakinumab-treatment-in-autoinflammatory-periodic-fever-syndromes-%e2%88%92-data-from-the-reliance-registry/