Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: A warning regarding safety of Interleukin 17 inhibitors (IL-17i) has been issued from data of randomized controlled trials (RCT) showing cases of new-onset inflammatory bowel diseases (NO-IBD). Real-world data are lacking. The objectives of the MISSIL (Maladies inflammatoires chroniques de l’Intestin SouS anti-IL 17) study are to describe NO-IBD associated with IL-17i, to identify risk factors, and to assess the incidence of NO-IBD in patients receiving treatment with IL-17i in France.
Methods: A French national registry was designed (MISSIL) to collect all cases of NO-IBD in patients treated with IL-17i from 2016 to 2019 in departments of rheumatology, dermatology and gastro-enterology, whatever the indication. A case–control study was performed with 3 controls per case randomly matched by gender, age (within 6 years), duration of disease (within 5 years) and underlying inflammatory disease from a database of previous studies of patients treated with IL-17i for spondyloarthritis and from centers participating in the MISSIL registry for psoriasis. NO-IBD events were analyzed using incidence rates (patient incidence rates per 100 patient-years – PY). We estimated the annual incidence rate of NO-IBD in patients treated with IL-17i. The numerator of the annual incidence rates consisted in the validated cases of NO-IBD from the MISSIL registry every year (from 2016 to 2019). The pharmaceutical firm provided its estimation of the annual number of patient-years (PY) treated with IL-17i for the denominator (from 2016 to 2019) in France.
Results: 31 cases of NO-IBD under secukinumab (SEK) were collected between January 2016 and December 2019: 27 patients treated for spondyloarthritis and 4 patients for psoriasis (Table 1). No case of NO-IBD was identified under ixekizumab. Mean age was 49.2 ± 14.6 years old and 14/31 were female; 14/24 were HLA-B27 positive, 10/25 had a radiographic sacroiliitis and 15/25 a MRI sacroiliitis. Only 4 were biological Disease-modifying antirheumatic drug (bDMARD)-naïve. The median time to onset of NO-IBD symptoms was 4.0 (1.5-7.5) months. The main symptoms were diarrhoea and loss of weight. Median CRP at the onset of symptoms was 68.0 mg/L (34.0-177.5). SEK was discontinued in all patients. Treatments received after SEK were: corticosteroids in 19 cases, infliximab in 14 cases, adalimumab in 7 cases, golimumab in 3 cases, ustekinumab in 7 cases and colectomy in 2 cases. The evolution was favourable with complete resolution (17/31), improvement (7/31) or stabilization (5/31). 2 patients died: one due to a massive myocardial infarction related to the severe undernutrition due to NO-IBD and one due to post-operative complications. The incidence of NO-IBD was 0.69/100 PY (7/1010) in 2016, 0.23/100 PY (11/4704) in 2017, 0.11/100 PY(7/6550) in 2018 and 0.08/100PY (6/7951). There was no independent risk factor for NO-IBD in the case-control study (Table 2).
Conclusion: The outcome of NO-IBD was favorable after SEK discontinuation and introduction of IBD treatment in the vast majority of patients. No independent risk factor associated with NO-IBD in patients initiating SEK was identified.
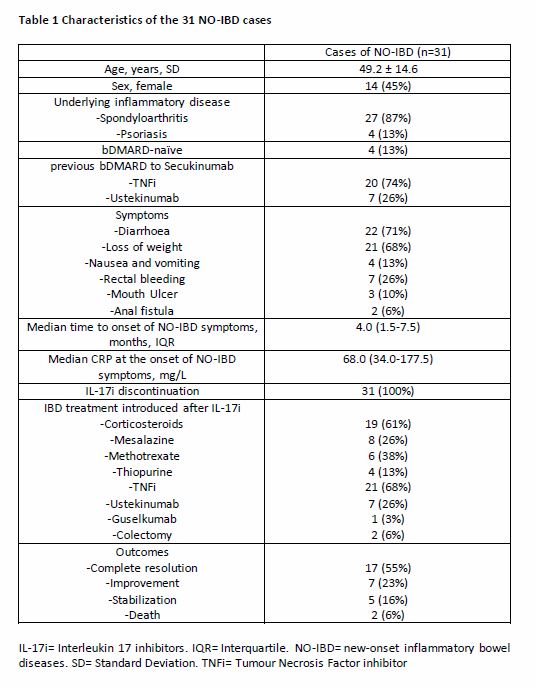 Table 1 Characteristics of the 31 NO-IBD cases IL-17i= Interleukin 17 inhibitors. IQR= Interquartile. NO-IBD= new-onset inflammatory bowel diseases. SD= Standard Deviation. TNFi= Tumour Necrosis Factor inhibitor
Table 1 Characteristics of the 31 NO-IBD cases IL-17i= Interleukin 17 inhibitors. IQR= Interquartile. NO-IBD= new-onset inflammatory bowel diseases. SD= Standard Deviation. TNFi= Tumour Necrosis Factor inhibitor
 Table 2 Risk Factor of NO-IBD receiving secukinumab bDMARD= biological Disease-modifying antirheumatic drug. csDMARD= conventional synthetic Disease-modifying antirheumatic drug. MTX= methotrexate
Table 2 Risk Factor of NO-IBD receiving secukinumab bDMARD= biological Disease-modifying antirheumatic drug. csDMARD= conventional synthetic Disease-modifying antirheumatic drug. MTX= methotrexate
To cite this abstract in AMA style:
Letarouilly J, Pariente B, Pham T, Acquacalda E, Banneville B, Barbarot S, Bauer E, Baudart P, Claudepierre P, Constantin A, Dernis E, Felten R, Gaudin P, Girard C, Gombert B, Goupille P, Guennoc X, Henry-Desailly I, Jullien D, Karimova E, Lanot S, Le Dantec L, Pascart T, Plastaras L, Sultan-Bichat N, Truchet X, Varin S, Wendling D, Gaboriau L, Staumont-Sallé D, Peyrin-Biroulet L, Flipo R. New-Onset Inflammatory Bowel Diseases Among IL-17 Inhibitors-Treated Patients: Results from the Case-Control MISSIL Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/new-onset-inflammatory-bowel-diseases-among-il-17-inhibitors-treated-patients-results-from-the-case-control-missil-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/new-onset-inflammatory-bowel-diseases-among-il-17-inhibitors-treated-patients-results-from-the-case-control-missil-study/
