Background/Purpose: Methotrexate (MTX) is often used as first-line DMARD-therapy in active psoriatic arthritis (PsA). Randomized clinical trials usually require treatment failure or intolerance of csDMARD/MTX before initiation of a biological treatment. The value of MTX in combination with bDMARDs is still unclear. We designed an investigator-initiated, randomized, placebo-controlled trial (IIT) in active PsA to examine if outcomes of treatment with ustekinumab (UST) in combination with MTX (either newly initiated or ongoing) were different from UST only (+Placebo; PBO). The aim of the trial was to evaluate non-inferiority of efficacy on arthritis (DAS28 at W24) and compare efficacy on PsA domains for UST+MTX vs UST+PBO.
Methods: A total of 186 patients with active PsA (defined as TJC≥4, SJC≥4 [68/66 joint count] and DAS28≥3.2) were screened for eligibility. 173 patients were randomized to UST+MTX (new or ongoing) or UST+PBO. Patients were stratified regarding their previous MTX therapy (blinded continuation of MTX or replacement of MTX with PBO [MTX-pretreated] or blinded newly-initiated MTX or PBO [MTX-naïve]). Demographic data and disease activity status (joint count [TJC/SJC], enthesitis [LEI], dactylitis count, PASI, BSA), QoL (EQ5D, DLQI) and function (HAQ) were compared between treatment groups. The primary outcome, non-inferiority of DAS28-ESR at week 24 for UST monotherapy (UST+PBO) vs UST+MTX, was assessed in concordance with a stratified van Elteren test with alpha=2.5% and tested by a non-inferiority margin of 12.5% according to the underlying stratified Mann-Whitney estimator (MWE). Additional outcomes included between-group differences in enthesitis, dactylitis, skin, QoL, and function.
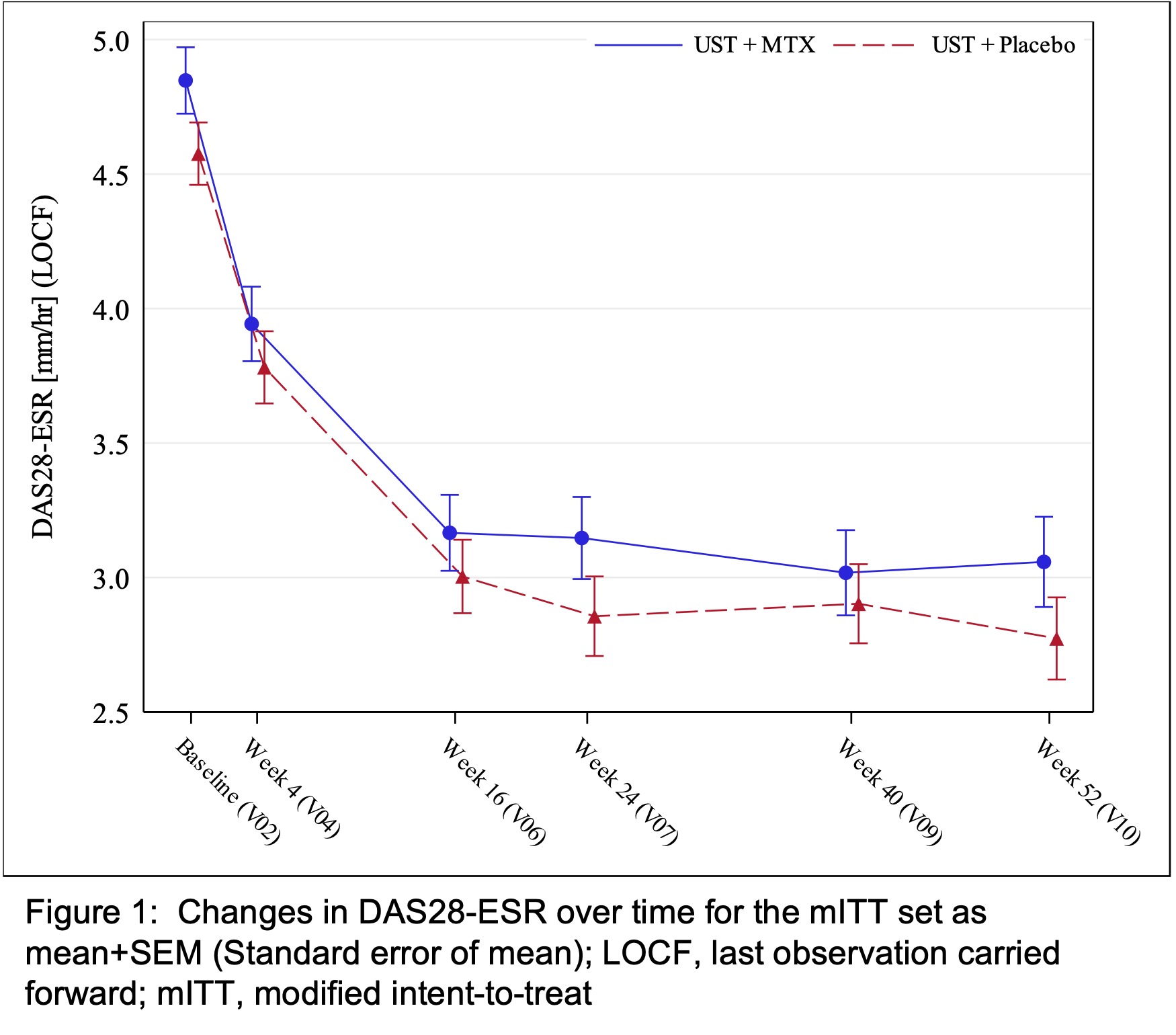
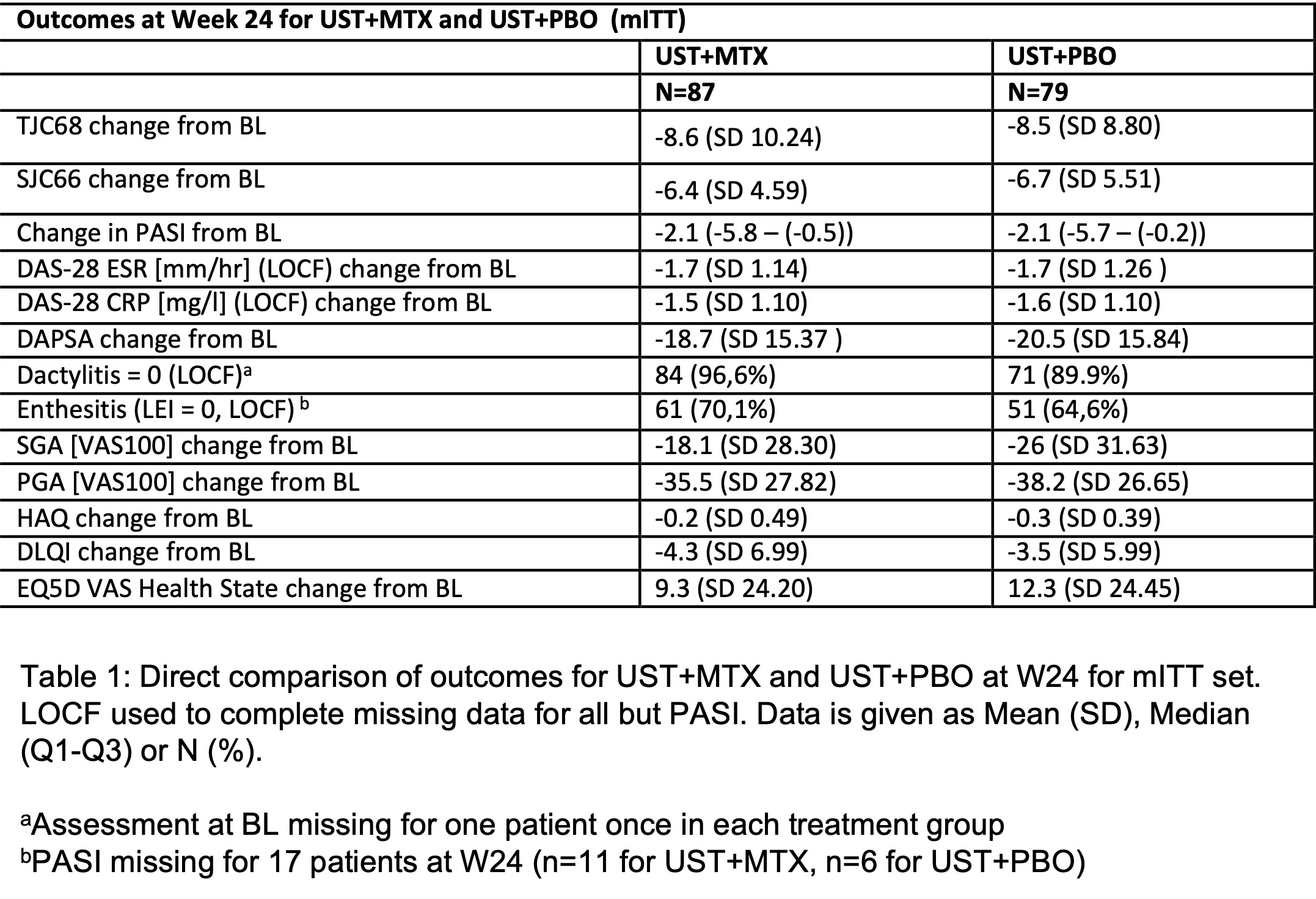
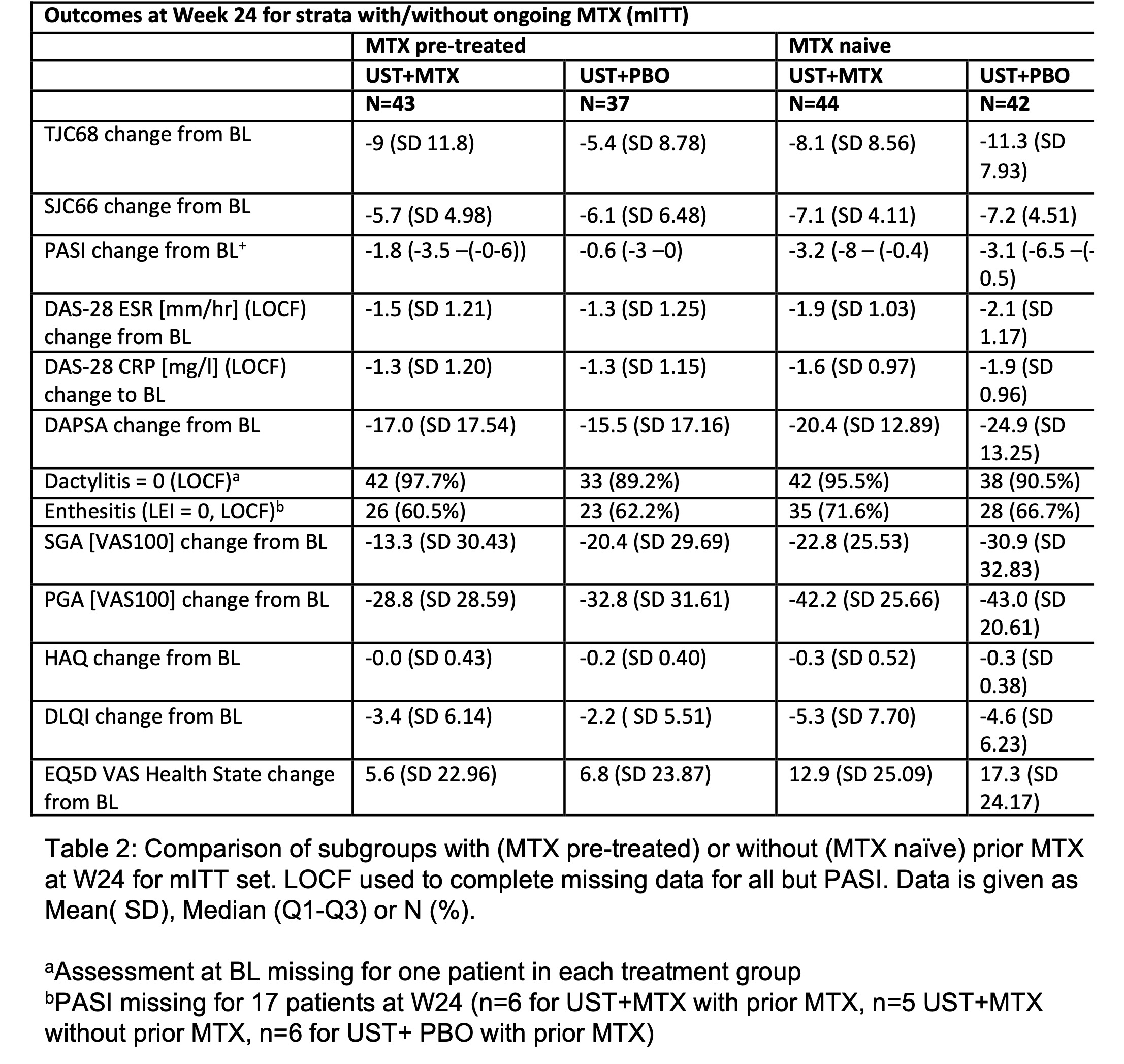
Results: BL data were well-balanced between treatment groups (UST+MTX, n=87; UST+PBO, n=79) including gender (42.5% vs 40.5% female) and mean values for age (49.2 vs 47.2 years), BMI (29.4 vs 28.9 kg/m2), SJC (8 vs 8), TJC (12 vs 12), DAS28-CRP (4.6 vs 4.4), DAPSA (36.7 vs 34.9), PASI (2.8 vs 2.4), enthesitis (LEI >0: 50.57% vs 50.63%), and other domains. BL differences were seen in dactylitis (24.1% vs 19.0%), BSA (2.9% vs 1.0%), and DLQI (8.6 vs 6.9). At week 24, mean DAS28-ESR decreased to 3.1 (SD 1.42) for UST+MTX vs 2.9 (SD 1.31) for UST+PBO with a stratified MWE for the treatment comparison of 0.5426 (95% CI 0.4545, 0.6307) demonstrating non-inferiority of UST+PBO (figure 1). Changes in other outcomes at week 24 were similar between groups (table 1). Within stratified subgroups, initiation or withdrawal of MTX did not impact UST efficacy (DAPSA change from BL: MTX pretreated, 17.0 vs 15.5 for UST+MTX and UST+PBO, respectively; MTX naïve, 20.4 vs 24.9). New initiation of UST (+PBO) resulted in the greatest improvements in DAS28, DAPSA, pain, HAQ, and EQ5D (table 3). 18% more AEs and the only two serious infections were reported for UST+MTX.
Conclusion: IL12/23 inhibition with UST is an effective treatment for active PsA independent of MTX use. Data from this IIT indicate that additional MTX has no positive impact on UST efficacy for arthritis, enthesitis, dactylitis, skin, QoL and function. Based on these data, there is no evidence to either add MTX or maintain ongoing MTX when starting UST.
To cite this abstract in AMA style:
Koehm M, Rossmanith T, Foldenauer A, Herrmann E, Kellner H, Kiltz U, Rech J, Burmester G, Kofler D, Brandt-Jürgens J, Jonetzko C, Burkhardt H, Behrens F. Neither Add-on nor Withdrawal of Methotrexate Impacts Efficacy of IL12/23 Inhibition in Active PsA: Data from a Multicenter Investigator-initiated Randomized Placebo-controlled Clinical Trial on Arthritis, Dactylitis, Enthesitis, Psoriasis, QoL and Function [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/neither-add-on-nor-withdrawal-of-methotrexate-impacts-efficacy-of-il12-23-inhibition-in-active-psa-data-from-a-multicenter-investigator-initiated-randomized-placebo-controlled-clinical-trial-on-arthr/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neither-add-on-nor-withdrawal-of-methotrexate-impacts-efficacy-of-il12-23-inhibition-in-active-psa-data-from-a-multicenter-investigator-initiated-randomized-placebo-controlled-clinical-trial-on-arthr/