Session Information
Date: Sunday, November 13, 2016
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment II: Clinical Trial Design
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: SLE clinical trials typically require that patients have either a positive ANA serology at a central laboratory during screening or have prior positive results. The definition of positive serology has varied among trials but, in all cases, a pre-specified titer of ANA has been sufficient to meet entry criteria. Studies that have allowed patients to enter with historically positive ANA serologies have been criticized because of concerns that these patients may not truly have lupus. Inconsistencies in the performance of ANAs and lack of standardization among the assays used in these trials may, however, contribute to the variability in ANA assay results.1 To further evaluate this issue, we have analyzed different ANA assays using samples collected in a Phase 2 randomized clinical trial which evaluated the efficacy and safety of an IL-6 mAb for the treatment of SLE.2 All potentially eligible subjects underwent a careful review of serology and clinical laboratory findings (ANA, anti-dsDNA, anti-Sm, anti-RNP, anti-SSA, anti-SSB, C3, and C4), medical history, and current lupus symptoms at screening. Those without a current positive ANA (Kallestad HEp-2 Cell Line Substrate) or anti-dsDNA by the central laboratory were also reviewed by an independent panel of lupus experts to confirm that the subject had both a positive historical ANA and clinically active lupus prior to randomization. Of the 183 patients enrolled, there were 43 (23.8%) who had <1:80 ANA titer at screening from the central laboratory.
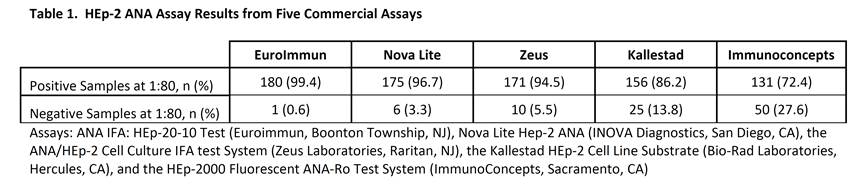
Methods: 181 samples collected at baseline in the clinical trial were evaluated using 5 commercially available HEp-2 ANA indirect fluorescent antibody (IFA) assays. All assays were performed according to the manufacturers’ protocols. Samples at 1:40 and 1:80 dilutions were run with each manufacturers’ positive and negative controls, and visualized using an EVOS FL Cell Imaging System.
Results: Overall, the EuroImmun assay resulted in the greatest percentage of positive results at a 1:80 dilution (99.4%). The Kallestad and Immunoconcepts assays had the lowest number of positive results of 86% and 72%, respectively. When combined, the EuroImmun and Nova Lite or Zeus assays yielded the maximum number of positive responses (100%). All 43 subjects with a <1:80 ANA titer during screening were ≥1:80 in 1 or more assays during this analysis.
Conclusion: In a well characterized lupus population, there was a large variation in the frequencies of positive samples depending on the ANA assay. These results suggest that careful consideration should be applied in the selection of an ANA assay for the purpose of establishing clinical trial eligibility. Preference may be given to assays that exhibit high sensitivity in patients with established SLE and using 2 sensitive assays may provide additional evidence for patient enrollment in SLE trials. 1. Copple SS et al. Am J Clin Pathol 2012;137:825-830. 2. Wallace D et al. ACR Annual Meeting 2014.
To cite this abstract in AMA style:
Pisetsky D, Thompson D, Wajdula J, Diehl A, Sridharan S. Negative Results of Antinuclear Antibody (ANA) Testing in Clinical Trials of Systemic Lupus Erythematosus (SLE) May be Due to Assay Variability [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/negative-results-of-antinuclear-antibody-ana-testing-in-clinical-trials-of-systemic-lupus-erythematosus-sle-may-be-due-to-assay-variability/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/negative-results-of-antinuclear-antibody-ana-testing-in-clinical-trials-of-systemic-lupus-erythematosus-sle-may-be-due-to-assay-variability/