Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
In our rheumatology practice, we noted an increased number of referrals for debilitating joint pain in inflammatory bowel disease (IBD) patients who received vedolizumab. Vedolizumab is a humanized monoclonal antibody targeting a4b7 integrin in the intestinal mucosa, FDA approved in 2014. The objective of this study was to assess for a potential link between arthralgias and subjects who received vedolizumab. Since joint symptoms are uncommonly coded by gastroenterologists, we hypothesized that applying natural language processing (NLP) to extract information from narrative notes, would detect an association that would not be observed using ICD9 codes alone.
Methods: We studied 10,742 IBD patients from a published and validated electronic medical record cohort from 2 large tertiary care centers. Vedolizumab users were identified by electronic prescriptions for infusions. Information on arthralgia was obtained using ³1 ICD9 codes for ‘arthralgia’ (719.4x), at any point during follow-up. We also processed notes for the concept arthralgia + subconcepts, e.g. ankle pain, with NLP arthralgia defined as ³1 mention of the concept ‘arthralgia’. To determine the performance characteristics of ICD9 and NLP for arthralgia, we randomly selected 100 subjects to establish gold standard cases of arthralgia. We tested the association between vedolizumab use and arthralgia by constructing logistic regression models adjusted by the potential confounders age, sex, IBD follow-up time and IBD treatments (Table 1).
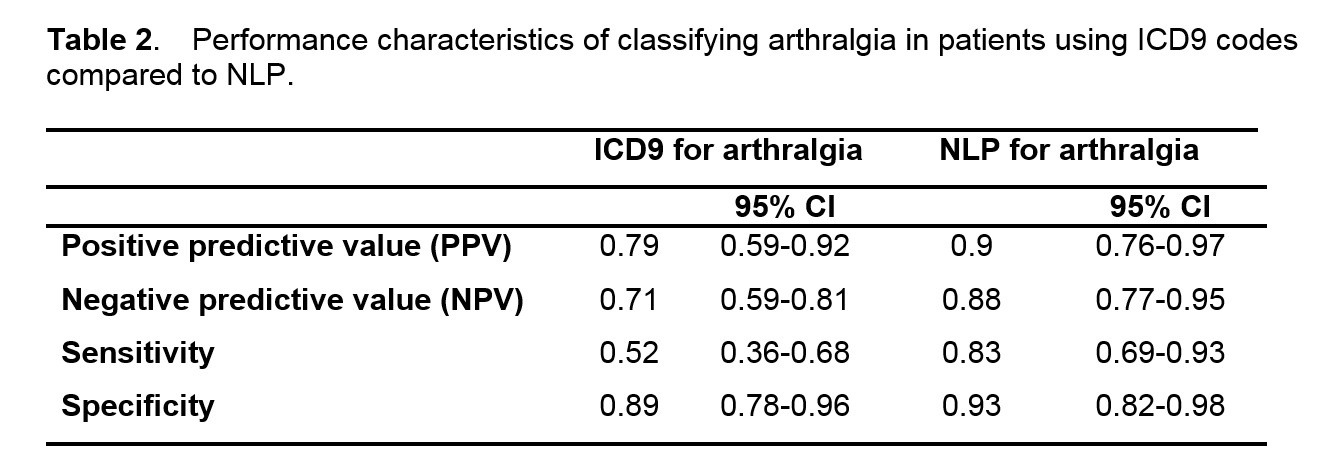
Results: We studied 10,742 subjects of which 3.5% received vedolizumab; clinical characteristics in Table 1. Compared to medical record review, NLP had a higher accuracy for arthralgia: PPV 0.90 vs 0.78 using ICD9 codes (Table 2). Using NLP, the prevalence of arthralgia was higher among IBD patients who received vedolizumab (77.1%) compared to those who did not (49.1%). After adjusting for potential confounders, there was no association between vedolizumab and the ICD9 code for arthralgia (OR 0.96, 95%CI 0.73-1.25). In contrast, we observed a significant association between vedolizumab and NLP for arthralgia after adjustment (OR 1.72, 95% CI 1.32-2.29).
Conclusion: Using NLP, we confirmed the clinical observation of an increased prevalence of arthralgias among patients who received vedolizumab. This signal would not have been detected using ICD9 codes alone. This study provided preliminary data for a potential association and future studies are needed to determine the exact timing of joint symptoms in relation to vedolizumab. 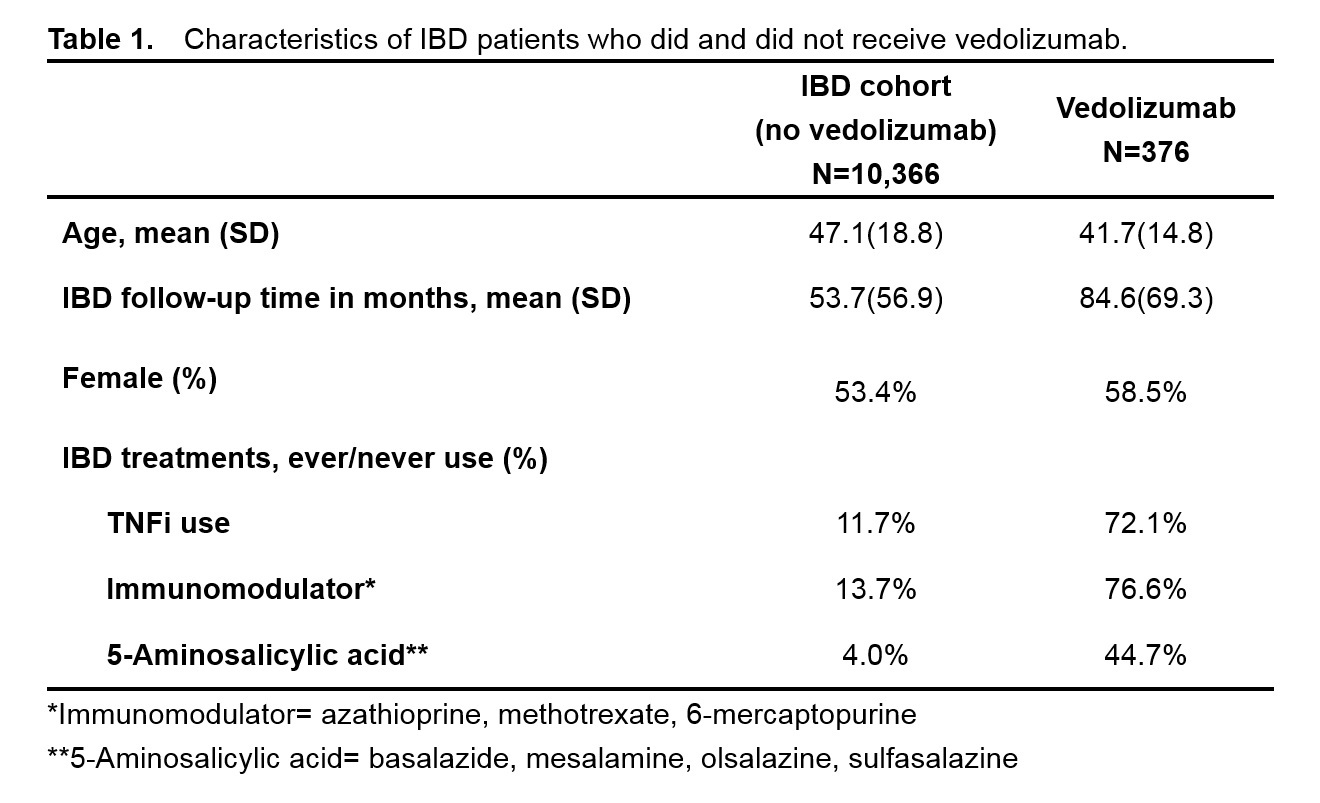
To cite this abstract in AMA style:
Cai T, Kane-Wanger G, Bond A, Cagan A, Murphy SN, Ananthakrishnan A, Liao K. Natural Language Processing to Rapidly Identify Potential Signals for Adverse Events Using Electronic Medical Record Data: Example of Arthralgias and Vedolizumab [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/natural-language-processing-to-rapidly-identify-potential-signals-for-adverse-events-using-electronic-medical-record-data-example-of-arthralgias-and-vedolizumab/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/natural-language-processing-to-rapidly-identify-potential-signals-for-adverse-events-using-electronic-medical-record-data-example-of-arthralgias-and-vedolizumab/