Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The American College of Rheumatology and National Quality Forum (NQF) recommend monitoring quality measures among rheumatoid arthritis (RA) patients. This study describes the proportion of RA patients within a large managed care population meeting the criteria of RA specific NQF quality measures.
Methods: Definitions of NQF RA quality measures 0054, 0589, 0590, 0592, 0597-0599, and 0601 were applied to claims data of a commercially-insured population from Optum Insight’s Clinformatics database for calendar years 2007-2011. NQF definitions may be found at www.qualityforum.org/Home.aspx.
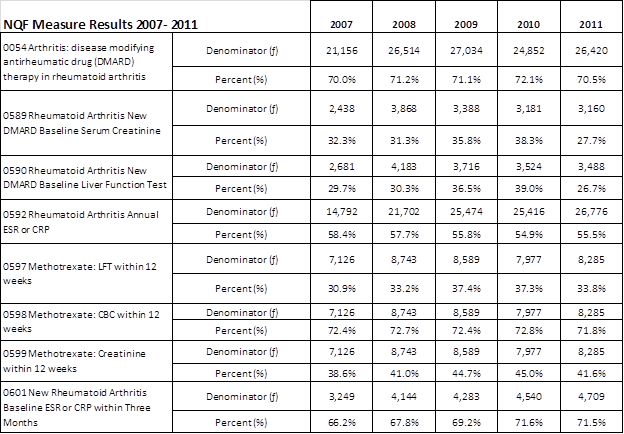
Results: Measure 0054 defines the proportion of RA patients treated with a DMARD in a defined measurement year. At each year studied, approximately 70% achieved this measure.
Measures 0589, 0590 pertain to laboratory monitoring of adult RA patients with a new DMARD in the measurement year. Measures 0589 (baseline serum creatine) and 0590 (baseline liver function test; LFT) were achieved by only one-third of patients. The proportion of patients achieving these measures appeared to increase annually between years 2007 and 2010 but declined during 2011.
Measures 0597, 0598 and 0599 were designed to monitor adult RA patients within 12 weeks of a new methotrexate (MTX) prescription. Approximately one-third of patients met the criteria for Measure 0597 (LFT within 12 weeks), and 40% met the criteria for 0599 (serum creatinine within 12 weeks). In contrast, approximately 70% of new MTX-treated patients met the criteria for 0598 (complete blood count within 12 weeks). Slight variations in the proportions of patients achieving these measures were observed from year to year.
Measures 0592 and 0601 pertain to monitoring of ESR or CRP in adult RA patients. Measure 0601 (proportion of newly diagnosed RA patients who received an ESR or CRP measure within 3 months of diagnosis) was achieved by approximately 70% of patients. Proportions of patients achieving this measure were improved slightly over time. However, annual ESR or CRP (0592) was achieved in slightly more than half the patients.
Conclusion: This analysis of a large national health plan suggests that between 30% and 70% of RA patients do not meet NQF quality measure criteria. Further studies are needed to understand: 1) the relationship between NQF measures and health outcomes and cost; 2) drivers of meeting NQF quality standards; and 3) interventions that improve NQF scores within health plans.
Disclosure:
R. Meyer,
Janssen Scientific Affairs, LLC,
3;
L. A. Ellis,
Janssen Scientific Affairs, LLC,
3;
S. C. Bolge,
Janssen Scientific Affairs, LLC,
3;
J. Tkacz,
Janssen Scientific Affairs, LLC,
5;
P. Kardel,
Janssen Scientific Affairs, LLC,
5;
C. Ruetsch,
Janssen Scientific Affairs, LLC,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/national-quality-forum-measures-among-rheumatoid-arthritis-patients-in-a-large-managed-care-population/